Molecular Imaging of Proteases in Cancer
- PMID: 20234801
- PMCID: PMC2838618
- DOI: 10.4137/cgm.s2814
Molecular Imaging of Proteases in Cancer
Abstract
Proteases play important roles during tumor angiogenesis, invasion, and metastasis. Various molecular imaging techniques have been employed for protease imaging: optical (both fluorescence and bioluminescence), magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), and positron emission tomography (PET). In this review, we will summarize the current status of imaging proteases in cancer with these techniques. Optical imaging of proteases, in particular with fluorescence, is the most intensively validated and many of the imaging probes are already commercially available. It is generally agreed that the use of activatable probes is the most accurate and appropriate means for measuring protease activity. Molecular imaging of proteases with other techniques (i.e. MRI, SPECT, and PET) has not been well-documented in the literature which certainly deserves much future effort. Optical imaging and molecular MRI of protease activity has very limited potential for clinical investigation. PET/SPECT imaging is suitable for clinical investigation; however the optimal probes for PET/SPECT imaging of proteases in cancer have yet to be developed. Successful development of protease imaging probes with optimal in vivo stability, tumor targeting efficacy, and desirable pharmacokinetics for clinical translation will eventually improve cancer patient management. Not limited to cancer, these protease-targeted imaging probes will also have broad applications in other diseases such as arthritis, atherosclerosis, and myocardial infarction.
Figures
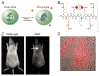
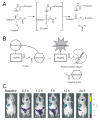

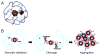

References
-
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. - PubMed
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. - PubMed
-
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. - PubMed
-
- Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5:785–99. - PubMed
-
- Garcia Boy R, Knapp EM, Eisenhut M, Haberkorn U, Mier W. Enzymes/transporters. Handb Exp Pharmacol. 2008:131–43. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

