Prostacyclin inhibits non-small cell lung cancer growth by a frizzled 9-dependent pathway that is blocked by secreted frizzled-related protein 1
- PMID: 20234818
- PMCID: PMC2838442
- DOI: 10.1593/neo.91690
Prostacyclin inhibits non-small cell lung cancer growth by a frizzled 9-dependent pathway that is blocked by secreted frizzled-related protein 1
Abstract
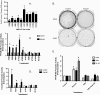
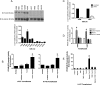
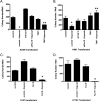
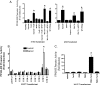
The goal of this study was to assess the ability of iloprost, an orally active prostacyclin analog, to inhibit transformed growth of human non-small cell lung cancer (NSCLC) and to define the mechanism of iloprost's tumor suppressive effects. In a panel of NSCLC cell lines, the ability of iloprost to inhibit transformed cell growth was not correlated with the expression of the cell surface receptor for prostacyclin, but instead was correlated with the presence of Frizzled 9 (Fzd 9) and the activation of peroxisome proliferator-activated receptor-gamma (PPARgamma). Silencing of Fzd 9 blocked PPARgamma activation by iloprost, and expression of Fzd 9 in cells lacking the protein resulted in iloprost's activation of PPARgamma and inhibition of transformed growth. Interestingly, soluble Frizzled-related protein-1, a well-known inhibitor of Wnt/Fzd signaling, also blocked the effects of iloprost and Fzd 9. Moreover, mice treated with iloprost had reduced lung tumors and increased Fzd 9 expression. These studies define a novel paradigm, linking the eicosanoid pathway and Wnt signaling. In addition, these data also suggest that prostacyclin analogs may represent a new class of therapeutic agents in the treatment of NSCLC where the restoration of noncanonical Wnt signaling maybe important for the inhibition of transformed cell growth.
Figures


Similar articles
-
Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor gamma.J Biol Chem. 2006 Sep 15;281(37):26943-50. doi: 10.1074/jbc.M604145200. Epub 2006 Jul 11. J Biol Chem. 2006. PMID: 16835228
-
Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation.J Biol Chem. 2005 May 20;280(20):19625-34. doi: 10.1074/jbc.M409392200. Epub 2005 Feb 10. J Biol Chem. 2005. PMID: 15705594
-
Sprouty-4 inhibits transformed cell growth, migration and invasion, and epithelial-mesenchymal transition, and is regulated by Wnt7A through PPARgamma in non-small cell lung cancer.Mol Cancer Res. 2010 Jun;8(6):833-43. doi: 10.1158/1541-7786.MCR-09-0400. Epub 2010 May 25. Mol Cancer Res. 2010. PMID: 20501643 Free PMC article.
-
Deregulation of Frizzled Receptors in Hepatocellular Carcinoma.Int J Mol Sci. 2018 Jan 21;19(1):313. doi: 10.3390/ijms19010313. Int J Mol Sci. 2018. PMID: 29361730 Free PMC article. Review.
-
Frizzled Receptors in Tumors, Focusing on Signaling, Roles, Modulation Mechanisms, and Targeted Therapies.Oncol Res. 2021 Mar 16;28(6):661-674. doi: 10.3727/096504020X16014648664459. Epub 2020 Sep 30. Oncol Res. 2021. PMID: 32998794 Free PMC article. Review.
Cited by
-
CD4 T cell knockout does not protect against kidney injury and worsens cancer.J Mol Med (Berl). 2016 Apr;94(4):443-55. doi: 10.1007/s00109-015-1366-z. Epub 2015 Dec 1. J Mol Med (Berl). 2016. PMID: 26620676
-
Dinosaurs and ancient civilizations: reflections on the treatment of cancer.Neoplasia. 2010 Dec;12(12):957-68. doi: 10.1593/neo.101588. Neoplasia. 2010. PMID: 21170260 Free PMC article.
-
Non-small-cell lung cancer: molecular targeted therapy and personalized medicine - drug resistance, mechanisms, and strategies.Pharmgenomics Pers Med. 2013 Apr 4;6:25-36. doi: 10.2147/PGPM.S26058. Print 2013. Pharmgenomics Pers Med. 2013. PMID: 23690695 Free PMC article.
-
Activation of PPARγ in myeloid cells promotes lung cancer progression and metastasis.Oncoimmunology. 2012 May 1;1(3):403-404. doi: 10.4161/onci.19309. Oncoimmunology. 2012. PMID: 22737631 Free PMC article.
-
The Second-Generation PGI2 Analogue Treprostinil Fails to Chemoprevent Tumors in a Murine Lung Adenocarcinoma Model.Cancer Prev Res (Phila). 2017 Nov;10(11):671-679. doi: 10.1158/1940-6207.CAPR-17-0050. Epub 2017 Aug 29. Cancer Prev Res (Phila). 2017. PMID: 28851689 Free PMC article.
References
-
- Michaud CM, Murray CJ, Bloom BR. Burden of disease—implications for future research. JAMA. 2001;285:535–539. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Honn KV, Cicone B, Skoff A. Prostacyclin: a potent antimetastatic agent. Science. 1981;212:1270–1272. - PubMed
-
- Keith RL, Miller YE, Hoshikawa Y, Moore MD, Gesell TL, Gao B, Malkinson AM, Golpon HA, Nemenoff RA, Geraci MW. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res. 2002;62:734–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
