Impact of metronomic UFT/cyclophosphamide chemotherapy and antiangiogenic drug assessed in a new preclinical model of locally advanced orthotopic hepatocellular carcinoma
- PMID: 20234820
- PMCID: PMC2838774
- DOI: 10.1593/neo.91872
Impact of metronomic UFT/cyclophosphamide chemotherapy and antiangiogenic drug assessed in a new preclinical model of locally advanced orthotopic hepatocellular carcinoma
Abstract
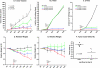
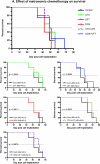
Hepatocellular carcinoma (HCC) is an intrinsically chemotherapy refractory malignancy. Development of effective therapeutic regimens would be facilitated by improved preclinical HCC models. Currently, most models consist of subcutaneous human tumor transplants in immunodeficient mice; however, these do not reproduce the extensive liver disease associated with HCC or metastasize. To address this deficiency, we developed an orthotopic model. Human HCC cells were transfected with the gene encoding secretable beta-subunit human choriogonadotropin (beta-hCG), which was used as a surrogate marker of tumor burden. The HCC cells were implanted into the left liver lobe of severe combined immunodeficient (SCID) mice, after which the efficacy of different therapies was evaluated on established, but liver-confined human Hep3B cell line HCC. Treatments included sorafenib or metronomic chemotherapy using cyclophosphamide (CTX), UFT, an oral 5-fluorouracil prodrug, or doxorubicin either alone or in various combinations, with or without an antiangiogenic agent, DC101, an anti-vascular endothelial growth factor receptor-2 antibody. Sorafenib inhibited tumor growth in a dose-dependent manner but caused severe weight loss in SCID mice, thus necessitating use of DC101 in subsequent experiments. Although less toxicity was observed using either single or doublet metronomic chemotherapy without any added antiangiogenic agent, none, provided survival benefit. In contrast, significantly improved overall survival was observed using various combinations of metronomic chemotherapy regimens such as UFT + CTX with DC101. In conclusion, using this model of liver-confined but advanced HCC suggests that the efficacy of a targeted antiangiogenic drug or metronomic chemotherapy can be mutually enhanced by concurrent combination treatment.
Figures

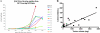


Similar articles
-
Development of a resistance-like phenotype to sorafenib by human hepatocellular carcinoma cells is reversible and can be delayed by metronomic UFT chemotherapy.Neoplasia. 2010 Nov;12(11):928-40. doi: 10.1593/neo.10804. Neoplasia. 2010. PMID: 21076618 Free PMC article.
-
Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy.Cancer Res. 2006 Apr 1;66(7):3386-91. doi: 10.1158/0008-5472.CAN-05-4411. Cancer Res. 2006. PMID: 16585158
-
Potent preclinical impact of metronomic low-dose oral topotecan combined with the antiangiogenic drug pazopanib for the treatment of ovarian cancer.Mol Cancer Ther. 2010 Apr;9(4):996-1006. doi: 10.1158/1535-7163.MCT-09-0960. Epub 2010 Apr 6. Mol Cancer Ther. 2010. PMID: 20371722 Free PMC article.
-
Sorafenib: in hepatocellular carcinoma.Drugs. 2008;68(2):251-8. doi: 10.2165/00003495-200868020-00007. Drugs. 2008. PMID: 18197728 Review.
-
Current status of hepatocellular carcinoma treatment in Japan: hepatic arterial infusion chemotherapy.Clin Drug Investig. 2012 Aug 8;32 Suppl 2:15-23. doi: 10.1007/BF03265493. Clin Drug Investig. 2012. PMID: 22873624 Review.
Cited by
-
The interconnectedness of cancer cell signaling.Neoplasia. 2011 Dec;13(12):1183-93. doi: 10.1593/neo.111746. Neoplasia. 2011. PMID: 22241964 Free PMC article.
-
Co-option of Liver Vessels and Not Sprouting Angiogenesis Drives Acquired Sorafenib Resistance in Hepatocellular Carcinoma.J Natl Cancer Inst. 2016 Apr 8;108(8):djw030. doi: 10.1093/jnci/djw030. Print 2016 Aug. J Natl Cancer Inst. 2016. PMID: 27059374 Free PMC article.
-
Wentilactone B induces G2/M phase arrest and apoptosis via the Ras/Raf/MAPK signaling pathway in human hepatoma SMMC-7721 cells.Cell Death Dis. 2013 Jun 6;4(6):e657. doi: 10.1038/cddis.2013.182. Cell Death Dis. 2013. PMID: 23744357 Free PMC article.
-
Longikaurin A, a natural ent-kaurane, induces G2/M phase arrest via downregulation of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells.Cell Death Dis. 2014 Mar 20;5(3):e1137. doi: 10.1038/cddis.2014.66. Cell Death Dis. 2014. PMID: 24651440 Free PMC article.
-
SNS01-T modulation of eIF5A inhibits B-cell cancer progression and synergizes with bortezomib and lenalidomide.Mol Ther. 2014 Sep;22(9):1643-52. doi: 10.1038/mt.2014.24. Epub 2014 Feb 26. Mol Ther. 2014. PMID: 24569836 Free PMC article.
References
-
- Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived—but they can be improved. Cancer Biol Ther. 2003;2(4 Suppl 1):S134–S139. - PubMed
-
- Man S, Munoz R, Kerbel RS. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev. 2007;26:737–747. - PubMed
-
- Kamb A. What's wrong with our cancer models? Nat Rev Drug Discov. 2005;4(2):161–165. - PubMed
-
- Burchill SA. What do, can and should we learn from models to evaluate potential anticancer agents? Future Oncol. 2006;2(2):201–211. - PubMed
-
- Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G, Kerbel RS. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66(7):3386–3391. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
