Cytometry in cell necrobiology revisited. Recent advances and new vistas
- PMID: 20235235
- PMCID: PMC2975392
- DOI: 10.1002/cyto.a.20889
Cytometry in cell necrobiology revisited. Recent advances and new vistas
Abstract
Over a decade has passed since publication of the last review on "Cytometry in cell necrobiology." During these years we have witnessed many substantial developments in the field of cell necrobiology such as remarkable advancements in cytometric technologies and improvements in analytical biochemistry. The latest innovative platforms such as laser scanning cytometry, multispectral imaging cytometry, spectroscopic cytometry, and microfluidic Lab-on-a-Chip solutions rapidly emerge as highly advantageous tools in cell necrobiology studies. Furthermore, we have recently gained substantial knowledge on alternative cell demise modes such as caspase-independent apoptosis-like programmed cell death (PCD), autophagy, necrosis-like PCD, or mitotic catastrophe, all with profound connotations to pathogenesis and treatment. Although detection of classical, caspase-dependent apoptosis is still the major ground for the advancement of cytometric techniques, there is an increasing demand for novel analytical tools to rapidly quantify noncanonical modes of cell death. This review highlights the key developments warranting a renaissance and evolution of cytometric techniques in the field of cell necrobiology.
Figures


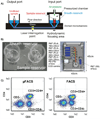

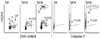
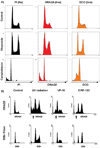
Similar articles
-
Rise of the micromachines: microfluidics and the future of cytometry.Methods Cell Biol. 2011;102:105-25. doi: 10.1016/B978-0-12-374912-3.00005-5. Methods Cell Biol. 2011. PMID: 21704837 Free PMC article.
-
Apoptosis and beyond: cytometry in studies of programmed cell death.Methods Cell Biol. 2011;103:55-98. doi: 10.1016/B978-0-12-385493-3.00004-8. Methods Cell Biol. 2011. PMID: 21722800 Free PMC article. Review.
-
Wormometry-on-a-chip: Innovative technologies for in situ analysis of small multicellular organisms.Cytometry A. 2011 Oct;79(10):799-813. doi: 10.1002/cyto.a.21070. Epub 2011 May 4. Cytometry A. 2011. PMID: 21548078 Review.
-
Szilard's dream.Nat Methods. 2005 Sep;2(9):648-9. doi: 10.1038/nmeth0905-648. Nat Methods. 2005. PMID: 16118633 No abstract available.
-
SYTO probes in the cytometry of tumor cell death.Cytometry A. 2008 Jun;73(6):496-507. doi: 10.1002/cyto.a.20535. Cytometry A. 2008. PMID: 18260152 Review.
Cited by
-
Multiparameter flow cytometry: advances in high resolution analysis.Immune Netw. 2013 Apr;13(2):43-54. doi: 10.4110/in.2013.13.2.43. Epub 2013 Apr 30. Immune Netw. 2013. PMID: 23700394 Free PMC article.
-
High-throughput dynamical analysis of dielectrophoretic frequency dispersion of single cells based on deflected flow streamlines.Anal Bioanal Chem. 2020 Jun;412(16):3847-3857. doi: 10.1007/s00216-020-02467-1. Epub 2020 Mar 4. Anal Bioanal Chem. 2020. PMID: 32128645 Free PMC article.
-
Self-aligned sequential lateral field non-uniformities over channel depth for high throughput dielectrophoretic cell deflection.Lab Chip. 2021 Mar 9;21(5):835-843. doi: 10.1039/d0lc01211d. Lab Chip. 2021. PMID: 33532812 Free PMC article.
-
Cytometry of apoptosis. Historical perspective and new advances.Exp Oncol. 2012 Oct;34(3):255-62. Exp Oncol. 2012. PMID: 23070010 Free PMC article. Review.
-
Real-time cell viability assays using a new anthracycline derivative DRAQ7®.Cytometry A. 2013 Feb;83(2):227-34. doi: 10.1002/cyto.a.22228. Epub 2012 Nov 16. Cytometry A. 2013. PMID: 23165976 Free PMC article.
References
-
- Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: Analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. - PubMed
-
- Darzynkiewicz Z, Bedner E, Traganos F. Difficulties and pitfalls in analysis of apoptosis. Methods Cell Biol. 2001;63:527–546. - PubMed
-
- Telford WG, Komoriya A, Packard BZ. Multiparametric analysis of apoptosis by flow and image cytometry. Methods Mol Biol. 2004;263:141–160. - PubMed
-
- Darzynkiewicz Z, Huang X, Okafuji M, King MA. Cytometric methods to detect apoptosis. Methods Cell Biol. 2004;75:307–341. - PubMed
-
- Bonetta L. Flow cytometry smaller and better. Nat Methods. 2005;2:785–795.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

