Adenoviral transduction of enterocytes and M-cells using in vitro models based on Caco-2 cells: the coxsackievirus and adenovirus receptor (CAR) mediates both apical and basolateral transduction
- PMID: 20235596
- PMCID: PMC2882510
- DOI: 10.1021/mp9001377
Adenoviral transduction of enterocytes and M-cells using in vitro models based on Caco-2 cells: the coxsackievirus and adenovirus receptor (CAR) mediates both apical and basolateral transduction
Abstract
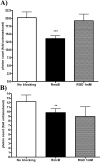
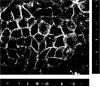
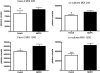
Understanding virus-cell interaction is a key to the design of successful gene delivery vectors. In the present study we investigated Ad5 transduction of enterocytes and M-cells utilizing differentiated Caco-2 cells and cocultures of Caco-2 cells with lymphocytes. Transduction inhibition studies showed that CAR is the major receptor mediating apical and basolateral virus entry in differentiated Caco-2 cells. Integrins and heparan sulfate glycosaminoglycans do not appear to play a significant role. Immunofluorescence localized CAR to sites of cell-cell contact, with staining mostly observed on the cell perimeter. Staining was observed even in nonpermeabilized monolayers, suggesting apical accessibility of the receptor. Cocultures with mouse Peyer's patch lymphocytes or Raji B human lymphocytes were more susceptible to transduction than Caco-2 cells, and the effects were dose-dependent. Similar to Caco-2 cells, CAR and not integrins mediated apical transduction. In conclusion, contrary to other epithelial cell lines, both apical and basolateral transduction of absorptive enterocytes and M-cells is mediated by binding to CAR. The coculture system can be used to study the interactions between M-cells and gene delivery vectors.
Figures









Similar articles
-
Restituting intestinal epithelial cells exhibit increased transducibility by adenoviral vectors.J Gene Med. 2006 Dec;8(12):1379-92. doi: 10.1002/jgm.981. J Gene Med. 2006. PMID: 17133338
-
Function and immunolocalization of overexpressed human intestinal H+/peptide cotransporter in adenovirus-transduced Caco-2 cells.AAPS PharmSci. 1999;1(3):E12. doi: 10.1208/ps010312. AAPS PharmSci. 1999. PMID: 11741208 Free PMC article.
-
Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice.Hum Gene Ther. 2003 May 20;14(8):777-87. doi: 10.1089/104303403765255165. Hum Gene Ther. 2003. PMID: 12804140
-
Molecular studies of the intestinal mucosal barrier physiopathology using cocultures of epithelial and immune cells: a technical update.Microbes Infect. 2000 Jul;2(9):1119-24. doi: 10.1016/s1286-4579(00)01266-1. Microbes Infect. 2000. PMID: 10967292 Review.
-
Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX.Virus Res. 2018 Sep 15;257:40-51. doi: 10.1016/j.virusres.2018.08.012. Epub 2018 Aug 17. Virus Res. 2018. PMID: 30125593 Review.
Cited by
-
Oral Vaccination with Replication-Competent Adenovirus in Mice Reveals Dissemination of the Viral Vaccine beyond the Gastrointestinal Tract.J Virol. 2019 Jun 14;93(13):e00237-19. doi: 10.1128/JVI.00237-19. Print 2019 Jul 1. J Virol. 2019. PMID: 30996103 Free PMC article.
-
The PDZ1 and PDZ3 domains of MAGI-1 regulate the eight-exon isoform of the coxsackievirus and adenovirus receptor.J Virol. 2012 Sep;86(17):9244-54. doi: 10.1128/JVI.01138-12. Epub 2012 Jun 20. J Virol. 2012. PMID: 22718816 Free PMC article.
-
The SRC family tyrosine kinase HCK and the ETS family transcription factors SPIB and EHF regulate transcytosis across a human follicle-associated epithelium model.J Biol Chem. 2013 Apr 12;288(15):10395-405. doi: 10.1074/jbc.M112.437475. Epub 2013 Feb 25. J Biol Chem. 2013. PMID: 23439650 Free PMC article.
-
Deciphering the M-cell niche: insights from mouse models on how microfold cells "know" where they are needed.Front Immunol. 2024 May 28;15:1400739. doi: 10.3389/fimmu.2024.1400739. eCollection 2024. Front Immunol. 2024. PMID: 38863701 Free PMC article. Review.
-
Firewalls Prevent Systemic Dissemination of Vectors Derived from Human Adenovirus Type 5 and Suppress Production of Transgene-Encoded Antigen in a Murine Model of Oral Vaccination.Front Cell Infect Microbiol. 2018 Jan 25;8:6. doi: 10.3389/fcimb.2018.00006. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29423380 Free PMC article.
References
-
- Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. - PubMed
-
- Walter E, Croyle MA, Davidson BL, Roessler BJ, Hilfinger JM, Amidon GL. Adenovirus mediated gene transfer to intestinal epithelial cells as a potential approach for oral delivery of peptides and proteins. J. Control. Release. 1997;46:75–87.
-
- Walter E, Croyle MA, Roessler BJ, Amidon GL. The absence of accessible vitronectin receptors in differentiated tissue hinders adenoviral-mediated gene transfer to the intestinal epithelium in vitro. Pharm. Res. 1997;14:1216–1222. - PubMed
-
- Croyle MA, Walter E, Janich S, Roessler BJ, Amidon GL. Role of integrin expression in adenovirus-mediated gene delivery to the intestinal epithelium. Hum. Gene Ther. 1998;9:561–573. - PubMed
-
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

