Modulation of cell surface expression of nonactivated cholecystokinin receptors using bivalent ligand-induced internalization
- PMID: 20235611
- PMCID: PMC3593351
- DOI: 10.1021/jm100135g
Modulation of cell surface expression of nonactivated cholecystokinin receptors using bivalent ligand-induced internalization
Abstract
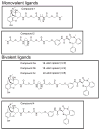
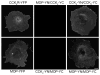
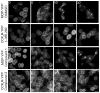
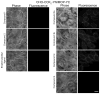
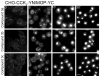
CCK(2) receptor antagonists potentiate pain relief by MOP receptor agonists. In an attempt to enhance this effect, we prepared bivalent ligands incorporating CCK(2) receptor antagonist and MOP receptor agonist pharmacophores. (9) Ligands with 16- to 22-atom spacers could simultaneously bind both receptors but provided no advantage in activity over individual ligands. We now examine the effect of these ligands on receptor internalization as a mechanism of receptor regulation. We prepared CHO cell lines expressing nonfluorescent halves (YN and YC) of yellow fluorescent protein attached to each receptor. Spatial approximation of constructs was needed to yield fluorescence. Monovalent MOP agonist 1 signaled normally and internalized the MOP receptor. Monovalent CCK(2) antagonist 2 did not stimulate receptor internalization. In the dual receptor-bearing cells, bivalent ligands 3a-c capable of simultaneously binding both receptors resulted in cell surface fluorescence and internalization of the fluorescent complex in a time- and temperature-dependent manner. Bivalent ligand 4 with spacer too short to occupy both receptors simultaneously yielded no signal. Receptor tethering with appropriate bivalent ligands can down-regulate signaling by moving a nonactivated receptor into the endocytic pathway.
Figures

Similar articles
-
Induced association of mu opioid (MOP) and type 2 cholecystokinin (CCK2) receptors by novel bivalent ligands.J Med Chem. 2009 Jan 22;52(2):247-58. doi: 10.1021/jm800174p. J Med Chem. 2009. PMID: 19113864 Free PMC article.
-
Estrogen and CCK1 receptor modification of mu-opioid receptor binding in the cortex of female rats.Brain Res. 2006 Feb 16;1073-1074:316-20. doi: 10.1016/j.brainres.2005.12.023. Epub 2006 Feb 10. Brain Res. 2006. PMID: 16472782
-
Visualization of G protein-coupled receptor trafficking with the aid of the green fluorescent protein. Endocytosis and recycling of cholecystokinin receptor type A.J Biol Chem. 1997 Jun 6;272(23):14817-24. doi: 10.1074/jbc.272.23.14817. J Biol Chem. 1997. PMID: 9169450
-
Strategies for design of non peptide CCK1R agonist/antagonist ligands.Curr Top Med Chem. 2007;7(12):1180-94. doi: 10.2174/156802607780960537. Curr Top Med Chem. 2007. PMID: 17584140 Review.
-
Applications of fluorescence in the characterization of the ligand-binding domain and activation of the cholecystokinin receptor.Pharmacol Toxicol. 2002 Dec;91(6):286-9. doi: 10.1034/j.1600-0773.2002.910604.x. Pharmacol Toxicol. 2002. PMID: 12688370 Review.
Cited by
-
Ligand-induced internalization of the type 1 cholecystokinin receptor independent of recognized signaling activity.Am J Physiol Cell Physiol. 2012 Feb 1;302(3):C615-27. doi: 10.1152/ajpcell.00193.2011. Epub 2011 Nov 2. Am J Physiol Cell Physiol. 2012. PMID: 22049215 Free PMC article.
-
Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction.ACS Chem Neurosci. 2013 Jan 16;4(1):110-21. doi: 10.1021/cn300072u. Epub 2012 Aug 11. ACS Chem Neurosci. 2013. PMID: 23336050 Free PMC article.
-
A Direct in Vivo Comparison of the Melanocortin Monovalent Agonist Ac-His-DPhe-Arg-Trp-NH2 versus the Bivalent Agonist Ac-His-DPhe-Arg-Trp-PEDG20-His-DPhe-Arg-Trp-NH2: A Bivalent Advantage.ACS Chem Neurosci. 2017 Jun 21;8(6):1262-1278. doi: 10.1021/acschemneuro.6b00399. Epub 2017 Feb 16. ACS Chem Neurosci. 2017. PMID: 28128928 Free PMC article.
-
Opioid bifunctional ligands from morphine and the opioid pharmacophore Dmt-Tic.Eur J Med Chem. 2011 Feb;46(2):799-803. doi: 10.1016/j.ejmech.2010.12.001. Epub 2010 Dec 8. Eur J Med Chem. 2011. PMID: 21216504 Free PMC article.
-
Development and in vitro characterization of a novel bifunctional μ-agonist/δ-antagonist opioid tetrapeptide.ACS Chem Biol. 2011 Dec 16;6(12):1375-81. doi: 10.1021/cb200263q. Epub 2011 Oct 11. ACS Chem Biol. 2011. PMID: 21958158 Free PMC article.
References
-
- Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. A bivalent ligand (KDAN-18) containing delta-antagonist and kappa-agonist pharmacophores bridges delta2 and kappa1 opioid receptor phenotypes. J Med Chem. 2005;48:1713–1716. - PubMed
-
- Erez M, Takemori AE, Portoghese PS. Narcotic antagonistic potency of bivalent ligands which contain beta-naltrexamine. Evidence for bridging between proximal recognition sites. J Med Chem. 1982;25:847–849. - PubMed
-
- Faris PL, Komisaruk BR, Watkins LR, Mayer DJ. Evidence for the neuropeptide cholecystokinin as an antagonist of opiate analgesia. Science. 1983;219:310–312. - PubMed
-
- Lu L, Huang M, Liu Z, Ma L. Cholecystokinin-B receptor antagonists attenuate morphine dependence and withdrawal in rats. NeuroReport. 2000;11:829–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

