The natural history of lymphangioleiomyomatosis: markers of severity, rate of progression and prognosis
- PMID: 20235883
- PMCID: PMC2883494
- DOI: 10.1089/lrb.2009.0024
The natural history of lymphangioleiomyomatosis: markers of severity, rate of progression and prognosis
Abstract
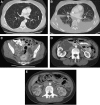
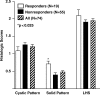
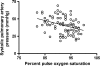
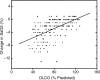
Lymphangioleiomyomatosis (LAM) is a multisystem disease of women, characterized by proliferation of abnormal smooth muscle-like cells (LAM cells) that can metastasize, leading to the formation of lung cysts, fluid-filled cystic structures in the axial lymphatics (e.g., lymphangioleiomyomas), and angiomyolipomas, benign tumors usually involving the kidneys, comprising LAM cells and adipocytes, intermixed with incompletely developed vascular structures. LAM occurs sporadically or in association with tuberous sclerosis complex, an autosomal dominant syndrome characterized by hamartoma-like tumor growths. LAM may present with progressive dyspnea, recurrent pneumothorax, chylothorax, or abdominal hemorrhage. Computed tomography scans show thin-walled cysts scattered throughout the lungs, abdominal angiomyolipomas, and lymphangioleiomyomas. Pulmonary function tests show reduced flow rates (FEV(1)) and diffusion capacity (DL(CO)). Exercise testing may reveal gas exchange abnormalities, ventilatory limitation, and hypoxemia, which can occur with near-normal lung function. Methods used to grade the severity of disease are the LAM histology score, semiquantitative and quantitative computer tomography, pulmonary function testing, and cardiopulmonary exercise testing. Currently, progression of disease is best assessed by serial measurements of FEV(1), DL(CO), and exercise performance. New quantitative radiographic techniques that may offer advantages over physiologic testing are now available. Several potential biomarkers, such as LAM cells in peripheral blood, urine, and chyle and chemokines, vascular endothelial growth factors, and matrix metalloproteinases, may be useful as diagnostic tools or markers of organ involvement, disease severity, and progression.
Figures
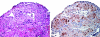



References
-
- Kitaichi M. Nishimura K. Harumi I. Izumi T. Pulmonary lymphangiomyomatosis: A report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med. 1995;151:527–533. - PubMed
-
- Sullivan EJ. Lymphangioleiomyomatosis: A review. Chest. 1998;114:1689–1703. - PubMed
-
- Chu SC. Horiba K. Usuki J, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999;115:1041–1052. - PubMed
-
- Urban TJ. Lazor R. Lacronique J, et al. Pulmonary lymphangioleiomyomatosis: A study of 69 patients. Medicine. 1999;78:321–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

