The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products
- PMID: 20235891
- PMCID: PMC2883528
- DOI: 10.1089/lrb.2009.0030
The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products
Abstract
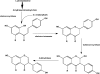
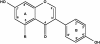
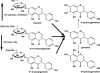
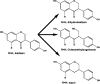
In this review of the chemistry, absorption, metabolism, and mechanisms of action of plant isoflavones, emphasis is placed on the isoflavones in soy and the food products derived from them. Soybeans have been part of food history in Asia for several millennia but did not reach the Americas and Europe until the eighteenth century. In the twentieth century, there was a tremendous increase in the cultivation of soybeans in the United States and more recently in South America. Soy foods have entered the U.S. food supply in ever-increasing amounts both in the form of traditional products (soy milk, tofu) and in more subtle ways in dairy and bread/cake products. The isoflavones in non-fermented foods are for the most part in the form of glycoside conjugates. These undergo changes due to different processing procedures. Isoflavones and their metabolites are well absorbed and undergo an enterohepatic circulation. They are often termed phytoestrogens because they bind to the estrogen receptors although weakly compared to physiologic estrogens. This estrogenicity is not the only mechanism by which isoflavones may have bioactivity-they inhibit tyrosine kinases, have antioxidant activity, bind to and activate peroxisome proliferator regulators alpha and gamma, inhibit enzymes in steroid biosynthesis, strongly influence natural killer cell function and the activation of specific T-cell subsets, and inhibit metastasis. These various properties may explain the much lower incidence of hormonally-dependent breast cancer in Asian populations compared to Americans and Europeans.
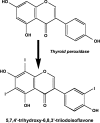
Figures
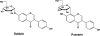
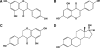
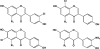
References
-
- Hymowitz T. Soybeans: The success story. In: Janick J., editor; Simon J.E., editor. Advances in new crops. Timber Press; Portland, OR: 1990. pp. 159–163.
-
- Sundquist WB. Cheng C-G. Norton GW. Measuring the Returns to Agricultural Experiment Station Research Expenditures for Corn, Wheat and Soybeans. Department of Agricultural and Applied Economics; 1980. pp. 1–43. Staff Paper P80-20.
-
- Rolfe BG. Flavones and isoflavones as inducing substances of legume nodulation. Biofactors. 1988;1:3–10. - PubMed
-
- Simonne AH. Smith M. Weaver DB. Vail T. Barnes S. Wei CI. Retention and changes of soy isoflavones and carotenoids in immature soybean seeds (Edamame) during processing. J Agric Food Chem. 2000;48:6061–6069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

