A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade?
- PMID: 20236142
- PMCID: PMC6493865
- DOI: 10.1111/j.1755-5949.2009.00116.x
A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade?
Abstract
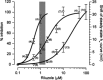
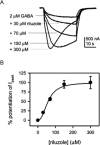
Amyotrophic lateral sclerosis (ALS) is a devastating and fatal neurodegenerative disease of adults which preferentially attacks the neuromotor system. Riluzole has been used as the only approved treatment for amyotrophic lateral sclerosis since 1995, but its mechanism(s) of action in slowing the progression of this disease remain obscure. Searching PubMed for "riluzole" found 705 articles published between January 1996 and June 2009. A systematic review of this literature found that riluzole had a wide range of effects on factors influencing neural activity in general, and the neuromotor system in particular. These effects occurred over a large dose range (<1 μM to >1 mM). Reported neural effects of riluzole included (in approximate ascending order of dose range): inhibition of persistent Na(+) current = inhibition of repetitive firing < potentiation of calcium-dependent K(+) current < inhibition of neurotransmitter release < inhibition of fast Na(+) current < inhibition of voltage-gated Ca(2+) current = promotion of neuronal survival or growth factors < inhibition of voltage-gated K(+) current = modulation of two-pore K(+) current = modulation of ligand-gated neurotransmitter receptors = potentiation of glutamate transporters. Only the first four of these effects commonly occurred at clinically relevant concentrations of riluzole (plasma levels of 1-2 μM with three- to four-fold higher concentrations in brain tissue). Treatment of human ALS patients or transgenic rodent models of ALS with riluzole most commonly produced a modest but significant extension of lifespan. Riluzole treatment was well tolerated in humans and animals. In animals, despite in vitro evidence that riluzole may inhibit rhythmic motor behaviors, in vivo administration of riluzole produced relatively minor effects on normal respiration parameters, but inhibited hypoxia-induced gasping. This effect may have implications for the management of hypoventilation and sleep-disordered breathing during end-stage ALS in humans.
© 2010 Blackwell Publishing Ltd.
Figures




References
-
- Domino EF, Unna KR, Kerwin J. Pharmacological properties of benzazoles. I. Relationship between structure and paralyzing action. J Pharmacol Exp Ther 1952;105:486–497. - PubMed
-
- Doble A. The pharmacology and mechanism of action of riluzole. Neurology 1996;47:233S–241S. - PubMed
-
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 2004;27:723–749. - PubMed
-
- Landwehrmeyer GB, Dubois B, de Yebenes JG, et al Riluzole in Huntington's disease: A 3‐year, randomized controlled study. Ann Neurol 2007;62:262–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

