Complementary transcriptomic, lipidomic, and targeted functional genetic analyses in cultured Drosophila cells highlight the role of glycerophospholipid metabolism in Flock House virus RNA replication
- PMID: 20236518
- PMCID: PMC2847973
- DOI: 10.1186/1471-2164-11-183
Complementary transcriptomic, lipidomic, and targeted functional genetic analyses in cultured Drosophila cells highlight the role of glycerophospholipid metabolism in Flock House virus RNA replication
Abstract
Background: Cellular membranes are crucial host components utilized by positive-strand RNA viruses for replication of their genomes. Published studies have suggested that the synthesis and distribution of membrane lipids are particularly important for the assembly and function of positive-strand RNA virus replication complexes. However, the impact of specific lipid metabolism pathways in this process have not been well defined, nor have potential changes in lipid expression associated with positive-strand RNA virus replication been examined in detail.
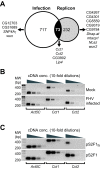
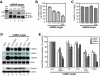
Results: In this study we used parallel and complementary global and targeted approaches to examine the impact of lipid metabolism on the replication of the well-studied model alphanodavirus Flock House virus (FHV). We found that FHV RNA replication in cultured Drosophila S2 cells stimulated the transcriptional upregulation of several lipid metabolism genes, and was also associated with increased phosphatidylcholine accumulation with preferential increases in lipid molecules with longer and unsaturated acyl chains. Furthermore, targeted RNA interference-mediated downregulation of candidate glycerophospholipid metabolism genes revealed a functional role of several genes in virus replication. In particular, we found that downregulation of Cct1 or Cct2, which encode essential enzymes for phosphatidylcholine biosynthesis, suppressed FHV RNA replication.
Conclusion: These results indicate that glycerophospholipid metabolism, and in particular phosphatidylcholine biosynthesis, plays an important role in FHV RNA replication. Furthermore, they provide a framework in which to further explore the impact of specific steps in lipid metabolism on FHV replication, and potentially identify novel cellular targets for the development of drugs to inhibit positive-strand RNA viruses.
Figures
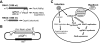


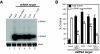

Similar articles
-
The cellular chaperone heat shock protein 90 facilitates Flock House virus RNA replication in Drosophila cells.J Virol. 2005 Jun;79(11):6827-37. doi: 10.1128/JVI.79.11.6827-6837.2005. J Virol. 2005. PMID: 15890922 Free PMC article.
-
The heat shock protein 70 cochaperone YDJ1 is required for efficient membrane-specific flock house virus RNA replication complex assembly and function in Saccharomyces cerevisiae.J Virol. 2008 Feb;82(4):2004-12. doi: 10.1128/JVI.02017-07. Epub 2007 Dec 5. J Virol. 2008. PMID: 18057252 Free PMC article.
-
Active complete in vitro replication of nodavirus RNA requires glycerophospholipid.Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11136-40. doi: 10.1073/pnas.89.23.11136. Proc Natl Acad Sci U S A. 1992. PMID: 1454791 Free PMC article.
-
Protein-protein interactions and glycerophospholipids in bromovirus and nodavirus RNA replication.Arch Virol Suppl. 1994;9:135-45. doi: 10.1007/978-3-7091-9326-6_14. Arch Virol Suppl. 1994. PMID: 8032245 Review.
-
Small RNAs and the control of transposons and viruses in Drosophila.Trends Microbiol. 2009 Apr;17(4):163-71. doi: 10.1016/j.tim.2009.01.003. Epub 2009 Mar 18. Trends Microbiol. 2009. PMID: 19299135 Review.
Cited by
-
An engineered mutant of a host phospholipid synthesis gene inhibits viral replication without compromising host fitness.J Biol Chem. 2019 Sep 20;294(38):13973-13982. doi: 10.1074/jbc.RA118.007051. Epub 2019 Jul 30. J Biol Chem. 2019. PMID: 31362985 Free PMC article.
-
Host Lipids in Positive-Strand RNA Virus Genome Replication.Front Microbiol. 2019 Feb 26;10:286. doi: 10.3389/fmicb.2019.00286. eCollection 2019. Front Microbiol. 2019. PMID: 30863375 Free PMC article. Review.
-
Lipidomic Profiling Reveals Distinct Differences in Sphingolipids Metabolic Pathway between Healthy Apis cerana cerana larvae and Chinese Sacbrood Disease.Insects. 2021 Aug 5;12(8):703. doi: 10.3390/insects12080703. Insects. 2021. PMID: 34442269 Free PMC article.
-
Viral rewiring of cellular lipid metabolism to create membranous replication compartments.Curr Opin Cell Biol. 2017 Aug;47:24-33. doi: 10.1016/j.ceb.2017.02.005. Epub 2017 Feb 24. Curr Opin Cell Biol. 2017. PMID: 28242560 Free PMC article. Review.
-
Antimicrobial Peptides as Potential Antiviral Factors in Insect Antiviral Immune Response.Front Immunol. 2020 Sep 2;11:2030. doi: 10.3389/fimmu.2020.02030. eCollection 2020. Front Immunol. 2020. PMID: 32983149 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

