Geographic and ecologic heterogeneity in elimination thresholds for the major vector-borne helminthic disease, lymphatic filariasis
- PMID: 20236528
- PMCID: PMC2848205
- DOI: 10.1186/1741-7007-8-22
Geographic and ecologic heterogeneity in elimination thresholds for the major vector-borne helminthic disease, lymphatic filariasis
Abstract
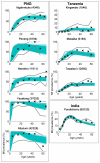
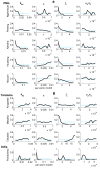
Background: Large-scale intervention programmes to control or eliminate several infectious diseases are currently underway worldwide. However, a major unresolved question remains: what are reasonable stopping points for these programmes? Recent theoretical work has highlighted how the ecological complexity and heterogeneity inherent in the transmission dynamics of macroparasites can result in elimination thresholds that vary between local communities. Here, we examine the empirical evidence for this hypothesis and its implications for the global elimination of the major macroparasitic disease, lymphatic filariasis, by applying a novel Bayesian computer simulation procedure to fit a dynamic model of the transmission of this parasitic disease to field data from nine villages with different ecological and geographical characteristics. Baseline lymphatic filariasis microfilarial age-prevalence data from three geographically distinct endemic regions, across which the major vector populations implicated in parasite transmission also differed, were used to fit and calibrate the relevant vector-specific filariasis transmission models. Ensembles of parasite elimination thresholds, generated using the Bayesian fitting procedure, were then examined in order to evaluate site-specific heterogeneity in the values of these thresholds and investigate the ecological factors that may underlie such variability
Results: We show that parameters of density-dependent functions relating to immunity, parasite establishment, as well as parasite aggregation, varied significantly between the nine different settings, contributing to locally varying filarial elimination thresholds. Parasite elimination thresholds predicted for the settings in which the mosquito vector is anopheline were, however, found to be higher than those in which the mosquito is culicine, substantiating our previous theoretical findings. The results also indicate that the probability that the parasite will be eliminated following six rounds of Mass Drug Administration with diethylcarbamazine and albendazole decreases markedly but non-linearly as the annual biting rate and parasite reproduction number increases.
Conclusions: This paper shows that specific ecological conditions in a community can lead to significant local differences in population dynamics and, consequently, elimination threshold estimates for lymphatic filariasis. These findings, and the difficulty of measuring the key local parameters (infection aggregation and acquired immunity) governing differences in transmission thresholds between communities, mean that it is necessary for us to rethink the utility of the current anticipatory approaches for achieving the elimination of filariasis both locally and globally.
Figures


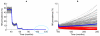
Similar articles
-
Bayesian calibration of simulation models for supporting management of the elimination of the macroparasitic disease, Lymphatic Filariasis.Parasit Vectors. 2015 Oct 22;8:522. doi: 10.1186/s13071-015-1132-7. Parasit Vectors. 2015. PMID: 26490350 Free PMC article.
-
Heterogeneous dynamics, robustness/fragility trade-offs, and the eradication of the macroparasitic disease, lymphatic filariasis.BMC Med. 2016 Jan 28;14:14. doi: 10.1186/s12916-016-0557-y. BMC Med. 2016. PMID: 26822124 Free PMC article.
-
Complex ecological dynamics and eradicability of the vector borne macroparasitic disease, lymphatic filariasis.PLoS One. 2008 Aug 6;3(8):e2874. doi: 10.1371/journal.pone.0002874. PLoS One. 2008. PMID: 18716676 Free PMC article.
-
Vector transmission heterogeneity and the population dynamics and control of lymphatic filariasis.Adv Exp Med Biol. 2010;673:13-31. doi: 10.1007/978-1-4419-6064-1_2. Adv Exp Med Biol. 2010. PMID: 20632527 Review.
-
Toward the elimination of lymphatic filariasis by 2020: treatment update and impact assessment for the endgame.Expert Rev Anti Infect Ther. 2013 Jul;11(7):723-31. doi: 10.1586/14787210.2013.811841. Expert Rev Anti Infect Ther. 2013. PMID: 23879610 Review.
Cited by
-
Elimination or Resurgence: Modelling Lymphatic Filariasis After Reaching the 1% Microfilaremia Prevalence Threshold.J Infect Dis. 2020 Jun 11;221(Suppl 5):S503-S509. doi: 10.1093/infdis/jiz647. J Infect Dis. 2020. PMID: 31853554 Free PMC article.
-
Accelerating river blindness elimination by supplementing MDA with a vegetation "slash and clear" vector control strategy: a data-driven modeling analysis.Sci Rep. 2019 Oct 24;9(1):15274. doi: 10.1038/s41598-019-51835-0. Sci Rep. 2019. PMID: 31649285 Free PMC article.
-
More Progress in Eliminating Transmission of Onchocerca volvulus and Wuchereria bancrofti in the Americas: A Portent of Global Eradication.Am J Trop Med Hyg. 2015 Dec;93(6):1128-1129. doi: 10.4269/ajtmh.15-0688. Epub 2015 Oct 26. Am J Trop Med Hyg. 2015. PMID: 26503272 Free PMC article. No abstract available.
-
Continental-scale, data-driven predictive assessment of eliminating the vector-borne disease, lymphatic filariasis, in sub-Saharan Africa by 2020.BMC Med. 2017 Sep 27;15(1):176. doi: 10.1186/s12916-017-0933-2. BMC Med. 2017. PMID: 28950862 Free PMC article.
-
Defining a prevalence level to describe the elimination of Lymphatic Filariasis (LF) transmission and designing monitoring & evaluating (M&E) programmes post the cessation of mass drug administration (MDA).PLoS Negl Trop Dis. 2020 Oct 12;14(10):e0008644. doi: 10.1371/journal.pntd.0008644. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33044958 Free PMC article.
References
-
- Sunish IP, Rajendran R, Mani TR, Munirathinam A, Tewari SC, Hiriyan J, Gajanana A, Satyanarayana K. Resurgence in filarial transmission after withdrawal of mass drug administration and the relationship between antigenaemia and microfilaraemia - a longitudinal study. Trop Med Int Health. 2002;7:59–69. doi: 10.1046/j.1365-3156.2002.00828.x. - DOI - PubMed

