Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain
- PMID: 20236533
- PMCID: PMC2845559
- DOI: 10.1186/1744-8069-6-16
Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain
Abstract
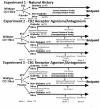
Background: Despite the frequency of diabetes mellitus and its relationship to diabetic peripheral neuropathy (DPN) and neuropathic pain (NeP), our understanding of underlying mechanisms leading to chronic pain in diabetes remains poor. Recent evidence has demonstated a prominent role of microglial cells in neuropathic pain states. One potential therapeutic option gaining clinical acceptance is the cannabinoids, for which cannabinoid receptors (CB) are expressed on neurons and microglia. We studied the accumulation and activation of spinal and thalamic microglia in streptozotocin (STZ)-diabetic CD1 mice and the impact of cannabinoid receptor agonism/antagonism during the development of a chronic NeP state. We provided either intranasal or intraperitoneal cannabinoid agonists/antagonists at multiple doses both at the initiation of diabetes as well as after establishment of diabetes and its related NeP state.
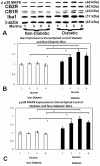
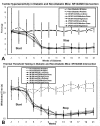
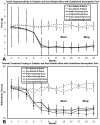
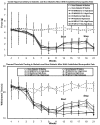
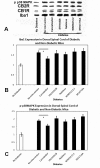
Results: Tactile allodynia and thermal hypersensitivity were observed over 8 months in diabetic mice without intervention. Microglial density increases were seen in the dorsal spinal cord and in thalamic nuclei and were accompanied by elevation of phosphorylated p38 MAPK, a marker of microglial activation. When initiated coincidentally with diabetes, moderate-high doses of intranasal cannabidiol (cannaboid receptor 2 agonist) and intraperitoneal cannabidiol attenuated the development of an NeP state, even after their discontinuation and without modification of the diabetic state. Cannabidiol was also associated with restriction in elevation of microglial density in the dorsal spinal cord and elevation in phosphorylated p38 MAPK. When initiated in an established DPN NeP state, both CB1 and CB2 agonists demonstrated an antinociceptive effect until their discontinuation. There were no pronociceptive effects demonstated for either CB1 or CB2 antagonists.
Conclusions: The prevention of microglial accumulation and activation in the dorsal spinal cord was associated with limited development of a neuropathic pain state. Cannabinoids demonstrated antinociceptive effects in this mouse model of DPN. These results suggest that such interventions may also benefit humans with DPN, and their early introduction may also modify the development of the NeP state.
Figures





Similar articles
-
Lidocaine attenuates the development of diabetic-induced tactile allodynia by inhibiting microglial activation.Anesth Analg. 2011 Oct;113(4):941-6. doi: 10.1213/ANE.0b013e31822827a2. Epub 2011 Jul 25. Anesth Analg. 2011. PMID: 21788310
-
1-(2',4'-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice.Neurobiol Dis. 2010 Jan;37(1):177-85. doi: 10.1016/j.nbd.2009.09.021. Epub 2009 Oct 3. Neurobiol Dis. 2010. PMID: 19804829
-
Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats.Eur J Pain. 2009 Sep;13(8):807-11. doi: 10.1016/j.ejpain.2008.09.010. Epub 2008 Oct 31. Eur J Pain. 2009. PMID: 18977160
-
Activated microglia in the spinal cord underlies diabetic neuropathic pain.Eur J Pharmacol. 2014 Apr 5;728:59-66. doi: 10.1016/j.ejphar.2014.01.057. Epub 2014 Feb 6. Eur J Pharmacol. 2014. PMID: 24508519 Review.
-
Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy.Prostaglandins Leukot Essent Fatty Acids. 2002 Feb-Mar;66(2-3):243-56. doi: 10.1054/plef.2001.0362. Prostaglandins Leukot Essent Fatty Acids. 2002. PMID: 12052040 Review.
Cited by
-
Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma 1 receptor.Mol Brain. 2018 Sep 17;11(1):51. doi: 10.1186/s13041-018-0395-2. Mol Brain. 2018. PMID: 30223868 Free PMC article.
-
Therapeutic Basis of Clinical Pain Modulation.Clin Transl Sci. 2015 Dec;8(6):848-56. doi: 10.1111/cts.12282. Epub 2015 May 11. Clin Transl Sci. 2015. PMID: 25962969 Free PMC article. Review.
-
Upregulation of TLR9 may contribute to activation of microglia and painful diabetic neuropathy via the p38 MAPK pathway in rats.Histol Histopathol. 2022 Jan;37(1):81-91. doi: 10.14670/HH-18-405. Epub 2021 Dec 10. Histol Histopathol. 2022. PMID: 34889455
-
Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice.Anesth Analg. 2011 Oct;113(4):947-50. doi: 10.1213/ANE.0b013e3182283486. Epub 2011 Jul 7. Anesth Analg. 2011. PMID: 21737705 Free PMC article.
-
Effects of Cannabidiol and Beta-Caryophyllene Alone or in Combination in a Mouse Model of Permanent Ischemia.Int J Mol Sci. 2021 Mar 11;22(6):2866. doi: 10.3390/ijms22062866. Int J Mol Sci. 2021. PMID: 33799861 Free PMC article.
References
-
- Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12(2):447–459. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

