Efficacy, safety and pharmacokinetic of once-daily boosted saquinavir (1500/100 mg) together with 2 nucleos(t)ide reverse transcriptase inhibitors in real life: a multicentre prospective study
- PMID: 20236544
- PMCID: PMC2847537
- DOI: 10.1186/1742-6405-7-5
Efficacy, safety and pharmacokinetic of once-daily boosted saquinavir (1500/100 mg) together with 2 nucleos(t)ide reverse transcriptase inhibitors in real life: a multicentre prospective study
Abstract
Background: Ritonavir-boosted saquinavir (SQVr) is nowadays regarded as an alternative antiretroviral drug probably due to several drawbacks, such as its high pill burden, twice daily dosing and the requirement of 200 mg ritonavir when given at the current standard 1000/100 mg bid dosing. Several once-daily SQVr dosing schemes have been studied with the 200 mg SQV old formulations, trying to overcome some of these disadvantages. SQV 500 mg strength tablets became available at the end of 2005, thus facilitating a once-daily regimen with fewer pills, although there is very limited experience with this formulation yet.
Methods: Prospective, multicentre study in which efficacy, safety and pharmacokinetics of a regimen of once-daily SQVr 1500/100 mg plus 2 NRTIs were evaluated under routine clinical care conditions in either antiretroviral-naïve patients or in those with no previous history of antiretroviral treatments and/or genotypic resistance tests suggesting SQV resistance. Plasma SQV trough levels were measured by HPLV-UV.
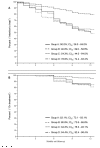
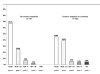
Results: Five hundred and fourteen caucasian patients were included (47.2% coinfected with hepatitis C and/or B virus; 7.8% with cirrhosis). Efficacy at 52 weeks (plasma RNA-HIV <50 copies/ml) was 67.7% (CI95: 63.6 - 71.7%) by intention-to-treat, and 92.2% (CI95: 89.8 - 94.6%) by on-treatment analysis. The reasons for failure were: dropout or loss to follow-up (18.4%), virological failure (7.8%), adverse events (3.1%), and other reasons (4.6%). The high rate of dropout may be explained by an enrollment and follow-up under routine clinical care condition, and a population with a significant number of drug users. The median SQV Cmin (n = 49) was 295 ng/ml (range, 53-2172). The only variable associated with virological failure in the multivariate analysis was adherence (OR: 3.36; CI95, 1.51-7.46, p = 0.003).
Conclusions: Our results suggests that SQVr (1500/100 mg) once-daily plus 2 NRTIs is an effective regimen, without severe clinical adverse events or hepatotoxicity, scarce lipid changes, and no interactions with methadone. All these factors and its once-daily administration suggest this regimen as an appropriate option in patients with no SQV resistance-associated mutations.
Figures
Similar articles
-
Clinical and pharmacokinetic data support once-daily low-dose boosted saquinavir (1,200 milligrams saquinavir with 100 milligrams ritonavir) in treatment-naive or limited protease inhibitor-experienced human immunodeficiency virus-infected patients.Antimicrob Agents Chemother. 2007 Jun;51(6):2035-42. doi: 10.1128/AAC.01136-06. Epub 2007 Mar 19. Antimicrob Agents Chemother. 2007. PMID: 17371813 Free PMC article. Clinical Trial.
-
The safety, efficacy, and pharmacokinetic profile of a switch in antiretroviral therapy to saquinavir, ritonavir, and atazanavir alone for 48 weeks and a switch in the saquinavir formulation.Clin Infect Dis. 2007 Jun 1;44(11):1475-83. doi: 10.1086/517507. Epub 2007 Apr 18. Clin Infect Dis. 2007. PMID: 17479946 Clinical Trial.
-
The 48-week efficacy of once-daily saquinavir/ritonavir in patients with undetectable viral load after 3 years of antiretroviral therapy.HIV Med. 2005 Mar;6(2):122-8. doi: 10.1111/j.1468-1293.2005.00274.x. HIV Med. 2005. PMID: 15807718 Clinical Trial.
-
Efficacy of low-dose boosted saquinavir once daily plus nucleoside reverse transcriptase inhibitors in pregnant HIV-1-infected women with a therapeutic drug monitoring strategy.Ther Drug Monit. 2007 Apr;29(2):171-6. doi: 10.1097/FTD.0b013e31803bb54e. Ther Drug Monit. 2007. PMID: 17417070
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
Cited by
-
Predictors of failure on second-line antiretroviral therapy with protease inhibitor mutations in Uganda.AIDS Res Ther. 2021 Apr 21;18(1):17. doi: 10.1186/s12981-021-00338-y. AIDS Res Ther. 2021. PMID: 33882938 Free PMC article.
References
-
- Dragsted UB, Gerstoft J, Youle M, Fox Z, Losso M, Benetucci J, Jayaweera DT, Rieger A, Bruun JN, Castagna A, Gazzard B, Walmsley S, Hill A, Lundgren JD. MaxCmin2 Trial Group. A randomized trial to evaluate lopinavir/ritonavir versus saquinavir/ritonavir in HIV-1-infected patients: the MaxCmin2 trial. Antivir Ther. 2005;10:735–743. - PubMed
-
- Walmsley S, Avihingsanon A, Slim J, Ward DJ, Ruxrungtham K, Brunetta J, Bredeek UF, Jayaweera D, Guittari CJ, Larson P, Schutz M, Raffi F. Gemini: a noninferiority study of saquinavir/ritonavir versus lopinavir/ritonavir as initial HIV-1 therapy in adults. J Acquir Immune Defic Syndr. 2009;50:367–374. doi: 10.1097/QAI.0b013e318198a815. - DOI - PubMed
-
- Roche Pharmaceuticals Invirase Prescribing Information, July. http://www.rocheusa.com/products/invirase/pi.pdf Accessed: 20 Jan 2010.
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. 2009. pp. 1–161.http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed: 15 Jan 2010.
-
- Lamotte C, Landman R, Peytavin G, Mentre F, Gerbe J, Brun-Vezinet F, Boue F, Spiridon G, Valantin MA, Michelet C, Farinotti R, Yeni P. Once-daily dosing of saquinavir soft-gel capsules and ritonavir combination in HIV-1-infected patients (IMEA015 study) Antivir Ther. 2004;9:247–256. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

