Increased BACE1 mRNA and noncoding BACE1-antisense transcript in sporadic inclusion-body myositis muscle fibers--possibly caused by endoplasmic reticulum stress
- PMID: 20236612
- PMCID: PMC2875146
- DOI: 10.1016/j.neulet.2010.03.023
Increased BACE1 mRNA and noncoding BACE1-antisense transcript in sporadic inclusion-body myositis muscle fibers--possibly caused by endoplasmic reticulum stress
Abstract
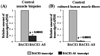
Sporadic inclusion-body myositis (s-IBM) is the most common muscle disease of older persons. Its muscle-fiber phenotype shares several molecular similarities with Alzheimer-disease (AD) brain, including increased AbetaPP, accumulation of amyloid-beta (Abeta), and increased BACE1 protein. Abeta42 is prominently increased in AD brain and within s-IBM fibers, and its oligomers are putatively toxic to both tissues--accordingly, minimizing Abeta42 production can be a therapeutic objective in both tissues. The pathogenic development of s-IBM is unknown, including the mechanisms of BACE1 protein increase. BACE1 is an enzyme essential for production from AbetaPP of Abeta42 and Abeta40, which are proposed to be detrimental within s-IBM muscle fibers. Novel noncoding BACE1-antisense (BACE1-AS) was recently shown (a) to be increased in AD brain, and (b) to increase BACE1 mRNA and BACE1 protein. We studied BACE1-AS and BACE1 transcripts by real-time PCR (a) in 10 s-IBM and 10 age-matched normal muscle biopsies; and (b) in our established ER-Stress-Human-Muscle-Culture-IBM Model, in which we previously demonstrated increased BACE1 protein. Our study demonstrated for the first time that (a) in s-IBM biopsies BACE1-AS and BACE1 transcripts were significantly increased, suggesting that their increased expression can be responsible for the increase of BACE1 protein; and (b) experimental induction of ER stress significantly increased both BACE1-AS and BACE1 transcripts, suggesting that ER stress can participate in their induction in s-IBM muscle. Accordingly, decreasing BACE1 through a targeted downregulation of its regulatory BACE1-AS, or reducing ER stress, might be therapeutic strategies in s-IBM, assuming that it would not impair any normal cellular functions of BACE1.
2010 Elsevier Ireland Ltd. All rights reserved.
Figures


Similar articles
-
NOGO is increased and binds to BACE1 in sporadic inclusion-body myositis and in A beta PP-overexpressing cultured human muscle fibers.Acta Neuropathol. 2007 Nov;114(5):517-26. doi: 10.1007/s00401-007-0281-y. Epub 2007 Aug 31. Acta Neuropathol. 2007. PMID: 17764014
-
BACE1 and BACE2 in pathologic and normal human muscle.Exp Neurol. 2003 Feb;179(2):150-8. doi: 10.1016/s0014-4886(02)00025-0. Exp Neurol. 2003. PMID: 12618121
-
Homocysteine-induced endoplasmic reticulum protein (Herp) is up-regulated in sporadic inclusion-body myositis and in endoplasmic reticulum stress-induced cultured human muscle fibers.J Neurochem. 2006 Mar;96(5):1491-9. doi: 10.1111/j.1471-4159.2006.03668.x. Epub 2006 Jan 25. J Neurochem. 2006. PMID: 16441512
-
Sporadic inclusion-body myositis: conformational multifactorial ageing-related degenerative muscle disease associated with proteasomal and lysosomal inhibition, endoplasmic reticulum stress, and accumulation of amyloid-β42 oligomers and phosphorylated tau.Presse Med. 2011 Apr;40(4 Pt 2):e219-35. doi: 10.1016/j.lpm.2010.11.024. Epub 2011 Mar 9. Presse Med. 2011. PMID: 21392932 Review.
-
Pathogenic considerations in sporadic inclusion-body myositis, a degenerative muscle disease associated with aging and abnormalities of myoproteostasis.J Neuropathol Exp Neurol. 2012 Aug;71(8):680-93. doi: 10.1097/NEN.0b013e31826183c8. J Neuropathol Exp Neurol. 2012. PMID: 22805774 Review.
Cited by
-
Long non-coding RNA BACE1-AS is an independent unfavorable prognostic factor in liver cancer.Oncol Lett. 2020 Nov;20(5):202. doi: 10.3892/ol.2020.12065. Epub 2020 Sep 8. Oncol Lett. 2020. PMID: 32963608 Free PMC article.
-
Long non-coding RNAs: novel targets for nervous system disease diagnosis and therapy.Neurotherapeutics. 2013 Oct;10(4):632-46. doi: 10.1007/s13311-013-0199-0. Neurotherapeutics. 2013. PMID: 23817781 Free PMC article. Review.
-
Updates on the Immunopathology in Idiopathic Inflammatory Myopathies.Curr Rheumatol Rep. 2021 Jul 1;23(7):56. doi: 10.1007/s11926-021-01017-7. Curr Rheumatol Rep. 2021. PMID: 34212266 Review.
-
Clinical, Histological, and Immunohistochemical Findings in Inclusion Body Myositis.Biomed Res Int. 2018 Jan 29;2018:5069042. doi: 10.1155/2018/5069042. eCollection 2018. Biomed Res Int. 2018. PMID: 29780824 Free PMC article.
-
BACE-1, PS-1 and sAPPβ Levels Are Increased in Plasma from Sporadic Inclusion Body Myositis Patients: Surrogate Biomarkers among Inflammatory Myopathies.Mol Med. 2016 Mar;21(1):817-823. doi: 10.2119/molmed.2015.00168. Epub 2015 Nov 3. Mol Med. 2016. PMID: 26552061 Free PMC article.
References
-
- Askanas V, Engel WK. Cultured normal and genetically abnormal human muscle. In: Rowland LP, Di Mauro S, editors. The Handbook of Clinical Neurology, Myopathies. Vol. 18. North Holland, Amsterdam: 1992. pp. 85–116.
-
- Askanas V, Engel WK, Alvarez RB. Enhanced detection of Congo-red positive amyloid deposits in muscle fibers of inclusion-body myositis and brain of Alzheimer’s disease using fluorescence technique. Neurology. 1993;43:1265–1267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

