Increased BACE1 mRNA and noncoding BACE1-antisense transcript in sporadic inclusion-body myositis muscle fibers--possibly caused by endoplasmic reticulum stress
- PMID: 20236612
- PMCID: PMC2875146
- DOI: 10.1016/j.neulet.2010.03.023
Increased BACE1 mRNA and noncoding BACE1-antisense transcript in sporadic inclusion-body myositis muscle fibers--possibly caused by endoplasmic reticulum stress
Abstract
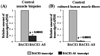
Sporadic inclusion-body myositis (s-IBM) is the most common muscle disease of older persons. Its muscle-fiber phenotype shares several molecular similarities with Alzheimer-disease (AD) brain, including increased AbetaPP, accumulation of amyloid-beta (Abeta), and increased BACE1 protein. Abeta42 is prominently increased in AD brain and within s-IBM fibers, and its oligomers are putatively toxic to both tissues--accordingly, minimizing Abeta42 production can be a therapeutic objective in both tissues. The pathogenic development of s-IBM is unknown, including the mechanisms of BACE1 protein increase. BACE1 is an enzyme essential for production from AbetaPP of Abeta42 and Abeta40, which are proposed to be detrimental within s-IBM muscle fibers. Novel noncoding BACE1-antisense (BACE1-AS) was recently shown (a) to be increased in AD brain, and (b) to increase BACE1 mRNA and BACE1 protein. We studied BACE1-AS and BACE1 transcripts by real-time PCR (a) in 10 s-IBM and 10 age-matched normal muscle biopsies; and (b) in our established ER-Stress-Human-Muscle-Culture-IBM Model, in which we previously demonstrated increased BACE1 protein. Our study demonstrated for the first time that (a) in s-IBM biopsies BACE1-AS and BACE1 transcripts were significantly increased, suggesting that their increased expression can be responsible for the increase of BACE1 protein; and (b) experimental induction of ER stress significantly increased both BACE1-AS and BACE1 transcripts, suggesting that ER stress can participate in their induction in s-IBM muscle. Accordingly, decreasing BACE1 through a targeted downregulation of its regulatory BACE1-AS, or reducing ER stress, might be therapeutic strategies in s-IBM, assuming that it would not impair any normal cellular functions of BACE1.
2010 Elsevier Ireland Ltd. All rights reserved.
Figures


References
-
- Askanas V, Engel WK. Cultured normal and genetically abnormal human muscle. In: Rowland LP, Di Mauro S, editors. The Handbook of Clinical Neurology, Myopathies. Vol. 18. North Holland, Amsterdam: 1992. pp. 85–116.
-
- Askanas V, Engel WK, Alvarez RB. Enhanced detection of Congo-red positive amyloid deposits in muscle fibers of inclusion-body myositis and brain of Alzheimer’s disease using fluorescence technique. Neurology. 1993;43:1265–1267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

