Abscisic acid ameliorates experimental IBD by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration
- PMID: 20236740
- PMCID: PMC2894983
- DOI: 10.1016/j.clnu.2010.02.009
Abscisic acid ameliorates experimental IBD by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration
Abstract
Background & aims: Abscisic acid (ABA) has shown effectiveness in ameliorating inflammation in obesity, diabetes and cardiovascular disease models. The objective of this study was to determine whether ABA prevents or ameliorates experimental inflammatory bowel disease (IBD).
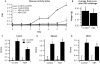
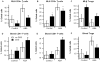
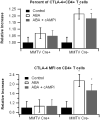
Methods: C57BL/6J mice were fed diets with or without ABA (100mg/kg) for 35 days prior to challenge with 2.5% dextran sodium sulfate (DSS). The severity of clinical disease was assessed daily. Colonic mucosal lesions were evaluated by histopathology, and cellular adhesion molecular and inflammatory markers were assayed by real-time quantitative PCR. Flow cytometry was used to quantify leukocyte populations in the blood, spleen, and mesenteric lymph nodes (MLN). The effect of ABA on cytotoxic T-lymphocyte antigen 4 (CTLA-4) expression in splenocytes was also investigated.
Results: ABA significantly ameliorated disease activity, colitis and reduced colonic leukocyte infiltration and inflammation. These improvements were associated with downregulation in vascular cell adhesion marker-1 (VCAM-1), E-selectin, and mucosal addressin adhesion marker-1 (MAdCAM-1) expression. ABA also increased CD4(+) and CD8(+) T-lymphocytes in blood and MLN and regulatory T cells in blood. In vitro, ABA increased CTLA-4 expression through a PPAR γ-dependent mechanism.
Conclusions: We conclude that ABA ameliorates gut inflammation by modulating T cell distribution and adhesion molecule expression.
Copyright © 2010 Elsevier Ltd and European Society for Clinical Nutrition and Metabolism. All rights reserved.
Conflict of interest statement
Figures


References
-
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–9. - PubMed
-
- Hanauer SB, Present DH. The state of the art in the management of inflammatory bowel disease. Rev Gastroenterol Disord. 2003;3(2):81–92. - PubMed
-
- Mackner LM, Sisson DP, Crandall WV. Review: psychosocial issues in pediatric inflammatory bowel disease. J Pediatr Psychol. 2004;29(4):243–257. - PubMed
-
- Bassaganya-Riera J, Skoneczka J, Kingston DGJ, Krishnan A, Misyak SA, Carter A, Pereira A, Guri AJ, Minorsky P, Turmakin R, et al. Mechanisms of action and medicinal applications of abscisic acid. Current Medicinal Chemistry. 2009 In press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

