Sphingosine 1-phosphate and sphingosine kinase are involved in a novel signaling pathway leading to acrosomal exocytosis
- PMID: 20236935
- PMCID: PMC2871498
- DOI: 10.1074/jbc.M109.072439
Sphingosine 1-phosphate and sphingosine kinase are involved in a novel signaling pathway leading to acrosomal exocytosis
Abstract
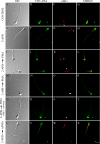
Regulated secretion is a central issue for the specific function of many cells; for instance, mammalian sperm acrosomal exocytosis is essential for egg fertilization. Sphingosine 1-phosphate is a bioactive sphingolipid that regulates crucial physiological processes. Here we report that this lipid triggers acrosomal exocytosis in human sperm by a mechanism involving a G(i)-coupled receptor. Real-time imaging showed a remarkable increase of cytosolic calcium upon activation with sphingosine 1-phosphate and pharmacological experiments indicate that the process requires extracellular calcium influx through voltage and store-operated calcium channels and efflux from intracellular stores through inositol 1,4,5-trisphosphate-sensitive calcium channels. Sphingosine 1-phosphate-induced exocytosis requires phospholipase C and protein kinase C activation. We investigated possible sources of the lipid. Western blot indicates that sphingosine kinase 1 is present in spermatozoa. Indirect immunofluorescence showed that phorbol ester, a potent protein kinase C activator that can also trigger acrosomal exocytosis, redistributes sphingosine kinase 1 to the acrosomal region. Functional assays showed that phorbol ester-induced exocytosis depends on the activation of sphingosine kinase 1. Furthermore, incorporation of (32)P to sphingosine demonstrates that cells treated with the phorbol ester increase their sphingosine kinase activity that yields sphingosine 1-phosphate. We present here the first evidence indicating that human spermatozoa produce sphingosine 1-phosphate when challenged with an exocytic stimulus. These observations point to a new role of sphingosine 1-phosphate in a signaling cascade that facilitates acrosome reaction providing some clues about novel lipid molecules involved in exocytosis.
Figures

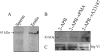


Similar articles
-
ADP ribosylation factor 6 (ARF6) promotes acrosomal exocytosis by modulating lipid turnover and Rab3A activation.J Biol Chem. 2015 Apr 10;290(15):9823-41. doi: 10.1074/jbc.M114.629006. Epub 2015 Feb 20. J Biol Chem. 2015. PMID: 25713146 Free PMC article.
-
Optimized protocols to analyze sphingosine-1-phosphate signal transduction pathways during acrosomal exocytosis in human sperm.Methods Mol Biol. 2012;874:99-128. doi: 10.1007/978-1-61779-800-9_9. Methods Mol Biol. 2012. PMID: 22528443
-
The pair ceramide 1-phosphate/ceramide kinase regulates intracellular calcium and progesterone-induced human sperm acrosomal exocytosis.Front Cell Dev Biol. 2023 Mar 31;11:1148831. doi: 10.3389/fcell.2023.1148831. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37065849 Free PMC article.
-
Regulatory mechanisms in acrosomal exocytosis.Rev Reprod. 1997 Sep;2(3):165-74. doi: 10.1530/ror.0.0020165. Rev Reprod. 1997. PMID: 9414480 Review.
-
Sphingosine kinase/sphingosine 1-phosphate signalling in central nervous system.Cell Signal. 2009 Jan;21(1):7-13. doi: 10.1016/j.cellsig.2008.07.011. Epub 2008 Jul 22. Cell Signal. 2009. PMID: 18694820 Review.
Cited by
-
ADP ribosylation factor 6 (ARF6) promotes acrosomal exocytosis by modulating lipid turnover and Rab3A activation.J Biol Chem. 2015 Apr 10;290(15):9823-41. doi: 10.1074/jbc.M114.629006. Epub 2015 Feb 20. J Biol Chem. 2015. PMID: 25713146 Free PMC article.
-
Metabolomics analysis of seminal plasma in infertile males with kidney-yang deficiency: a preliminary study.Evid Based Complement Alternat Med. 2015;2015:892930. doi: 10.1155/2015/892930. Epub 2015 Apr 7. Evid Based Complement Alternat Med. 2015. PMID: 25945117 Free PMC article.
-
Application of High-Throughput Assays to Examine Phospho-Modulation of the Late Steps of Regulated Exocytosis.High Throughput. 2017 Nov 13;6(4):17. doi: 10.3390/ht6040017. High Throughput. 2017. PMID: 29479054 Free PMC article.
-
Metabolites involved in cellular communication among human cumulus-oocyte-complex and sperm during in vitro fertilization.Reprod Biol Endocrinol. 2015 Nov 9;13:123. doi: 10.1186/s12958-015-0118-9. Reprod Biol Endocrinol. 2015. PMID: 26553294 Free PMC article.
-
Sphingosine-1-phosphate rapidly increases cortisol biosynthesis and the expression of genes involved in cholesterol uptake and transport in H295R adrenocortical cells.Mol Cell Endocrinol. 2012 Jan 2;348(1):165-75. doi: 10.1016/j.mce.2011.08.003. Epub 2011 Aug 16. Mol Cell Endocrinol. 2012. PMID: 21864647 Free PMC article.
References
-
- Spiegel S., English D., Milstien S. (2002) Trends Cell Biol. 12, 236–242 - PubMed
-
- Taha T. A., Argraves K. M., Obeid L. M. (2004) Biochim. Biophys. Acta 1682, 48–55 - PubMed
-
- Yatomi Y. (2006) Curr. Pharm. Des. 12, 575–587 - PubMed
-
- Rosen H., Goetzl E. J. (2005) Nat. Rev. Immunol. 5, 560–570 - PubMed
-
- Sanchez T., Hla T. (2004) J. Cell Biochem. 92, 913–922 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

