Activation of vascular endothelial growth factor receptor 2 in a cellular model of loricrin keratoderma
- PMID: 20236940
- PMCID: PMC2871486
- DOI: 10.1074/jbc.M109.056424
Activation of vascular endothelial growth factor receptor 2 in a cellular model of loricrin keratoderma
Abstract
Loricrin is a major constituent of the epidermal cornified cell envelope. Recently, heterozygous loricrin gene mutations have been identified in two dominantly inherited skin diseases, Vohwinkel syndrome with ichthyosis and progressive symmetric erythrokeratoderma, collectively termed loricrin keratoderma. We generated stable HaCaT cell lines that express wild-type (WT) loricrin and a mutant form found in Vohwinkel syndrome with ichthyosis, using an ecdysone-inducible promoter system. The cells expressing the mutant loricrin grew more rapidly than those expressing WT loricrin after induction for 5 days. Confocal immunofluorescence microscopy revealed that phospho-Akt occurred in the nucleolus where the mutant loricrin was also located. The level of activity of Akt kinase was about nine times higher in cells with the mutant than in those with WT loricrin. ERK1/2, the epidermal growth factor receptor, vascular endothelial growth factor (VEGF) receptor 2 and Stat3 were all phosphorylated in cells with the mutant loricrin. The docking proteins, Gab1 and c-Cbl, were also tyrosine-phosphorylated in these cells. Furthermore, chromatin immunoprecipitation assays showed that Stat3 protein bound to the VEGF promoter in cells with the mutant. Thus, this study suggests that VEGF release and the subsequent activation of VEGF receptor 2 link loricrin gene mutations to rapid cell proliferation in a cellular model of loricrin keratoderma.
Figures






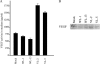

References
-
- Kalinin A., Marekov L. N., Steinert P. M. (2001) J. Cell Sci. 114, 3069–3070 - PubMed
-
- Kalinin A. E., Kajava A. V., Steinert P. M. (2002) BioEssays 24, 789–800 - PubMed
-
- Yoneda K., Hohl D., McBride O. W., Wang M., Cehrs K. U., Idler W. W., Steinert P. M. (1992) J. Biol. Chem. 267, 18060–18066 - PubMed
-
- Yoneda K., McBride O. W., Korge B. P., Kim I. G., Steinert P. M. (1992) J. Dermatol. 19, 761–764 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

