18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis
- PMID: 20237034
- PMCID: PMC4141648
- DOI: 10.2967/jnumed.108.060459
18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis
Abstract
PET imaging is useful for evaluating locally advanced primary breast cancer. Expression of specific molecular markers in these cancers, such as estrogen receptor (ER), progesterone receptor (PR), and HER2 status, has direct prognostic and therapeutic implications in patient management. This study aimed to determine whether a relationship exists between tumor glucose use and important molecular markers in invasive breast cancer. For our purposes, tumor glucose use is quantified by the PET-derived parameter maximum standardized uptake value (SUV).
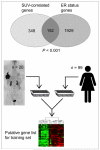
Methods: Breast tumors from 36 patients were excised and examined histologically after PET. ER, PR, and HER2 status were determined for all lesions histopathologically. In addition, genomewide expression for a subset of 20 tumors was analyzed using the human genome U133A oligonucleotide microarray.
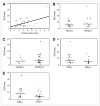
Results: A significant association was found between estrogen ER status and lesion SUV. ER-negative tumors (n = 17; median SUV, 8.5) demonstrated a significantly higher maximum SUV than did ER-positive tumors (n = 19; median SUV, 4.0) (P < 0.001). No significant association existed between SUV and PR status, HER2/neu status, lymph node involvement, or tumor size. Unsupervised hierarchic clustering of the 20 genetically profiled cancers segregated tumor samples into 2 primary groups of 10 patients each, largely corresponding to ER status.
Conclusion: In locally invasive primary breast cancer, ER-negative tumors display higher (18)F-FDG uptake than ER-positive tumors. Microarray analysis confirms these data and identifies genes associated with increased glucose use as measured by PET. These genes significantly overlap those of a previously validated ER-status molecular phenotype. These preliminary data support a growing body of evidence that ER-positive and ER-negative breast cancers have distinct disease-specific patterns. Further validation prospectively and with larger numbers will be required to establish a robust molecular signature for metabolic uptake and patterns of aggressive behavior in advanced breast cancer.
Figures

Similar articles
-
Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for disease characterization.Cancer. 2008 Mar 1;112(5):995-1000. doi: 10.1002/cncr.23226. Cancer. 2008. PMID: 18098228 Clinical Trial.
-
The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG uptake of primary breast cancer lesions.J Nucl Med. 2007 Aug;48(8):1266-72. doi: 10.2967/jnumed.106.037440. Epub 2007 Jul 13. J Nucl Med. 2007. PMID: 17631558
-
Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer.J Nucl Med. 2013 Oct;54(10):1697-702. doi: 10.2967/jnumed.112.113373. Epub 2013 Aug 22. J Nucl Med. 2013. PMID: 23970364 Free PMC article. Clinical Trial.
-
Current applications of PET imaging of sex hormone receptors with a fluorinated analogue of estradiol or of testosterone.Q J Nucl Med Mol Imaging. 2015 Mar;59(1):4-17. Epub 2015 Feb 19. Q J Nucl Med Mol Imaging. 2015. PMID: 25693420 Review.
-
Using nuclear medicine imaging in clinical practice: update on PET to guide treatment of patients with metastatic breast cancer.Oncology (Williston Park). 2014 May;28(5):424-30. Oncology (Williston Park). 2014. PMID: 25004657 Review.
Cited by
-
Correlation between Histopathological Prognostic Tumor Characteristics and [18F]FDG Uptake in Corresponding Metastases in Newly Diagnosed Metastatic Breast Cancer.Diagnostics (Basel). 2024 Feb 14;14(4):416. doi: 10.3390/diagnostics14040416. Diagnostics (Basel). 2024. PMID: 38396455 Free PMC article.
-
A review on immunohistochemical and histopathologic validation in PET-CT findings with consideration to microRNAs.Med Pharm Rep. 2019 Oct;92(4):337-345. doi: 10.15386/mpr-1341. Epub 2019 Oct 25. Med Pharm Rep. 2019. PMID: 31750432 Free PMC article. Review.
-
Non-invasive tumor genotyping using radiogenomic biomarkers, a systematic review and oncology-wide pathway analysis.Oncotarget. 2018 Apr 13;9(28):20134-20155. doi: 10.18632/oncotarget.24893. eCollection 2018 Apr 13. Oncotarget. 2018. PMID: 29732009 Free PMC article. Review.
-
Spectrum of the Breast Lesions With Increased 18F-FDG Uptake on PET/CT.Clin Nucl Med. 2016 Jul;41(7):543-57. doi: 10.1097/RLU.0000000000001203. Clin Nucl Med. 2016. PMID: 26975010 Free PMC article.
-
Personalized medicine: a new option for nuclear medicine and molecular imaging in the third millennium.Eur J Nucl Med Mol Imaging. 2017 Apr;44(4):563-566. doi: 10.1007/s00259-017-3616-5. Eur J Nucl Med Mol Imaging. 2017. PMID: 28083691 No abstract available.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. - PubMed
-
- Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. - PubMed
-
- Wahl RL, Cody RL, Hutchins GD, Mudgett EE. Primary and metastatic breast carcinoma: initial clinical evaluation with PET with the radiolabeled glucose analogue 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1991;179:765–770. - PubMed
-
- Avril N, Bense S, Ziegler SI, et al. Breast imaging with fluorine-18-FDG PET: quantitative image analysis. J Nucl Med. 1997;38:1186–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
