Cleavage of influenza virus hemagglutinin by airway proteases TMPRSS2 and HAT differs in subcellular localization and susceptibility to protease inhibitors
- PMID: 20237084
- PMCID: PMC2876594
- DOI: 10.1128/JVI.00140-10
Cleavage of influenza virus hemagglutinin by airway proteases TMPRSS2 and HAT differs in subcellular localization and susceptibility to protease inhibitors
Abstract
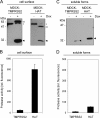
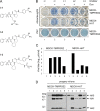
Proteolytic cleavage of the influenza virus surface glycoprotein hemagglutinin (HA) by host cell proteases is crucial for infectivity and virus spread. The proteases HAT (human airway trypsin-like protease) and TMPRSS2 (transmembrane protease serine S1 member 2) known to be present in the human airways were previously identified as proteases that cleave HA. We studied subcellular localization of HA cleavage and cleavage inhibition of seasonal influenza virus A/Memphis/14/96 (H1N1) and pandemic virus A/Hamburg/5/2009 (H1N1) in MDCK cells that express HAT and TMPRSS2 under doxycycline-induced transcriptional activation. We made the following observations: (i) HA is cleaved by membrane-bound TMPRSS2 and HAT and not by soluble forms released into the supernatant; (ii) HAT cleaves newly synthesized HA before or during the release of progeny virions and HA of incoming viruses prior to endocytosis at the cell surface, whereas TMPRSS2 cleaves newly synthesized HA within the cell and is not able to support the proteolytic activation of HA of incoming virions; and (iii) cleavage activation of HA and virus spread in TMPRSS2- and HAT-expressing cells can be suppressed by peptide mimetic protease inhibitors. The further development of these inhibitors could lead to new drugs for influenza treatment.
Figures




References
-
- Afar, D. E., I. Vivanco, R. S. Hubert, J. Kuo, E. Chen, D. C. Saffran, A. B. Raitano, and A. Jakobovits. 2001. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 61:1686-1692. - PubMed
-
- Böttcher, E., C. Freuer, T. Steinmetzer, H. D. Klenk, and W. Garten. 2009. MDCK cells that express proteases TMPRSS2 and HAT provide a cell system to propagate influenza viruses in the absence of trypsin and to study cleavage of HA and its inhibition. Vaccine 27:6324-6329. - PubMed
-
- Boycott, R., H. D. Klenk, and M. Ohuchi. 1994. Cell tropism of influenza virus mediated by hemagglutinin activation at the stage of virus entry. Virology 203:313-319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

