A regulated complex of the scaffolding proteins PDZK1 and EBP50 with ezrin contribute to microvillar organization
- PMID: 20237154
- PMCID: PMC2861611
- DOI: 10.1091/mbc.e10-01-0008
A regulated complex of the scaffolding proteins PDZK1 and EBP50 with ezrin contribute to microvillar organization
Abstract
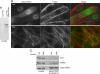
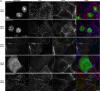
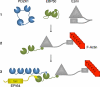
PDZK1 and ezrin, radixin, moesin binding phosphoprotein 50 kDa (EBP50) are postsynaptic density 95/disc-large/zona occludens (PDZ)-domain-containing scaffolding proteins found in the apical microvilli of polarized epithelial cells. Binary interactions have been shown between the tail of PDZK1 and the PDZ domains of EBP50, as well as between EBP50 and the membrane-cytoskeletal linking protein ezrin. Here, we show that these molecules form a regulated ternary complex in vitro and in vivo. Complex formation is cooperative because ezrin positively influences the PDZK1/EBP50 interaction. Moreover, the interaction of PDZK1 with EBP50 is enhanced by the occupancy of EBP50's adjacent PDZ domain. The complex is further regulated by location, because PDZK1 shuttles from the nucleus in low confluence cells to microvilli in high confluence cells, and this regulates the formation of the PDZK1/EBP50/ezrin complex in vivo. Knockdown of EBP50 decreases the presence of microvilli, a phenotype that can be rescued by EBP50 re-expression or expression of a PDZK1 chimera that is directly targeted to ezrin. Thus, when appropriately located, PDZK1 can provide a function necessary for microvilli formation normally provided by EBP50. By entering into the ternary complex, PDZK1 can both enhance the scaffolding at the apical membrane as well as augment EBP50's role in microvilli formation.
Figures



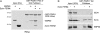



References
-
- Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 2006;8:348–357. - PubMed
-
- Bretscher A., Edwards K., Fehon R. G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002;3:586–599. - PubMed
-
- Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. - PubMed
-
- Doyle D. A., Lee A., Lewis J., Kim E., Sheng M., MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

