Yip1A structures the mammalian endoplasmic reticulum
- PMID: 20237155
- PMCID: PMC2861614
- DOI: 10.1091/mbc.e09-12-1002
Yip1A structures the mammalian endoplasmic reticulum
Abstract
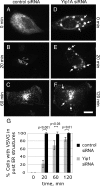
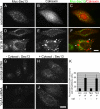
The structure of the endoplasmic reticulum (ER) undergoes highly regulated changes in specialized cell types. One frequently observed type of change is its reorganization into stacked and concentrically whorled membranes, but the underlying mechanisms and functional relevance for cargo export are unknown. Here, we identify Yip1A, a conserved membrane protein that cycles between the ER and early Golgi, as a key mediator of ER organization. Yip1A depletion led to restructuring of the network into multiple, micrometer-sized concentric whorls. Membrane stacking and whorl formation coincided with a marked slowing of coat protein (COP)II-mediated protein export. Furthermore, whorl formation driven by exogenous expression of an ER protein with no role in COPII function also delayed cargo export. Thus, the slowing of protein export induced by Yip1A depletion may be attributed to a proximal role for Yip1A in regulating ER network dispersal. The ER network dispersal function of Yip1A was blocked by alteration of a single conserved amino acid (E95K) in its N-terminal cytoplasmic domain. These results reveal a conserved Yip1A-mediated mechanism for ER membrane organization that may serve to regulate cargo exit from the organelle.
Figures



Similar articles
-
Yip1A regulates the COPI-independent retrograde transport from the Golgi complex to the ER.J Cell Sci. 2009 Jul 1;122(Pt 13):2218-27. doi: 10.1242/jcs.043414. Epub 2009 Jun 9. J Cell Sci. 2009. PMID: 19509059
-
Identification of discrete sites in Yip1A necessary for regulation of endoplasmic reticulum structure.PLoS One. 2013;8(1):e54413. doi: 10.1371/journal.pone.0054413. Epub 2013 Jan 14. PLoS One. 2013. PMID: 23342155 Free PMC article.
-
A membrane protein enriched in endoplasmic reticulum exit sites interacts with COPII.J Biol Chem. 2001 Oct 26;276(43):40008-17. doi: 10.1074/jbc.M106189200. Epub 2001 Aug 6. J Biol Chem. 2001. PMID: 11489904
-
New insights into the structural mechanisms of the COPII coat.Traffic. 2010 Mar;11(3):303-10. doi: 10.1111/j.1600-0854.2009.01026.x. Epub 2009 Dec 7. Traffic. 2010. PMID: 20070605 Review.
-
ER-to-Golgi transport: form and formation of vesicular and tubular carriers.Biochim Biophys Acta. 2005 Jul 10;1744(3):304-15. doi: 10.1016/j.bbamcr.2005.03.003. Epub 2005 Mar 23. Biochim Biophys Acta. 2005. PMID: 15979504 Review.
Cited by
-
The ALS8 protein VAPB interacts with the ER-Golgi recycling protein YIF1A and regulates membrane delivery into dendrites.EMBO J. 2013 Jul 17;32(14):2056-72. doi: 10.1038/emboj.2013.131. Epub 2013 Jun 4. EMBO J. 2013. PMID: 23736259 Free PMC article.
-
Multi-step down-regulation of the secretory pathway in mitosis: a fresh perspective on protein trafficking.Bioessays. 2013 May;35(5):462-71. doi: 10.1002/bies.201200144. Epub 2013 Mar 12. Bioessays. 2013. PMID: 23494566 Free PMC article.
-
Functional characterisation of the YIPF protein family in mammalian cells.Histochem Cell Biol. 2017 Apr;147(4):439-451. doi: 10.1007/s00418-016-1527-3. Epub 2016 Dec 20. Histochem Cell Biol. 2017. PMID: 27999994
-
Irradiation-induced protein inactivation reveals Golgi enzyme cycling to cell periphery.J Cell Sci. 2012 Feb 15;125(Pt 4):973-80. doi: 10.1242/jcs.094441. Epub 2012 Mar 15. J Cell Sci. 2012. PMID: 22421362 Free PMC article.
-
BPIFB3 Regulates Endoplasmic Reticulum Morphology To Facilitate Flavivirus Replication.J Virol. 2020 Apr 16;94(9):e00029-20. doi: 10.1128/JVI.00029-20. Print 2020 Apr 16. J Virol. 2020. PMID: 32102874 Free PMC article.
References
-
- Ahluwalia N., Bergeron J. J., Wada I., Degen E., Williams D. B. The p88 molecular chaperone is identical to the endoplasmic reticulum membrane protein, calnexin. J. Biol. Chem. 1992;267:10914–10918. - PubMed
-
- Amarilio R., Ramachandran S., Sabanay H., Lev S. Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J. Biol. Chem. 2005;280:5934–5944. - PubMed
-
- Anderson R. G., Orci L., Brown M. S., Garcia-Segura L. M., Goldstein J. L. Ultrastructural analysis of crystalloid endoplasmic reticulum in UT-1 cells and its disappearance in response to cholesterol. J. Cell Sci. 1983;63:1–20. - PubMed
-
- Aridor M., Balch W. E. Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J. Biol. Chem. 2000;275:35673–35676. - PubMed
-
- Barr F. A., Puype M., Vandekerckhove J., Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

