Endogenous RhoG is rapidly activated after epidermal growth factor stimulation through multiple guanine-nucleotide exchange factors
- PMID: 20237158
- PMCID: PMC2861620
- DOI: 10.1091/mbc.e09-09-0809
Endogenous RhoG is rapidly activated after epidermal growth factor stimulation through multiple guanine-nucleotide exchange factors
Erratum in
- Mol Biol Cell. 2011 Jul 15;22(14):2659
Abstract
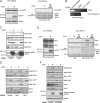
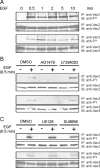
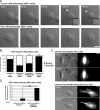
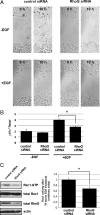
RhoG is a member of the Rac-like subgroup of Rho GTPases and has been linked to a variety of different cellular functions. Nevertheless, many aspects of RhoG upstream and downstream signaling remain unclear; in particular, few extracellular stimuli that modulate RhoG activity have been identified. Here, we describe that stimulation of epithelial cells with epidermal growth factor leads to strong and rapid activation of RhoG. Importantly, this rapid activation was not observed with other growth factors tested. The kinetics of RhoG activation after epidermal growth factor (EGF) stimulation parallel the previously described Rac1 activation. However, we show that both GTPases are activated independently of one another. Kinase inhibition studies indicate that the rapid activation of RhoG and Rac1 after EGF treatment requires the activity of the EGF receptor kinase, but neither phosphatidylinositol 3-kinase nor Src kinases. By using nucleotide-free RhoG pull-down assays and small interfering RNA-mediated knockdown studies, we further show that guanine-nucleotide exchange factors (GEFs) of the Vav family mediate EGF-induced rapid activation of RhoG. In addition, we found that in certain cell types the recently described RhoG GEF PLEKHG6 can also contribute to the rapid activation of RhoG after EGF stimulation. Finally, we present results that show that RhoG has functions in EGF-stimulated cell migration and in regulating EGF receptor internalization.
Figures






Similar articles
-
The Src homology 3 domain-containing guanine nucleotide exchange factor is overexpressed in high-grade gliomas and promotes tumor necrosis factor-like weak inducer of apoptosis-fibroblast growth factor-inducible 14-induced cell migration and invasion via tumor necrosis factor receptor-associated factor 2.J Biol Chem. 2013 Jul 26;288(30):21887-97. doi: 10.1074/jbc.M113.468686. Epub 2013 Jun 17. J Biol Chem. 2013. PMID: 23775076 Free PMC article.
-
Phosphorylation and activation of the Rac1 and Cdc42 GEF Asef in A431 cells stimulated by EGF.J Cell Sci. 2008 Aug 15;121(Pt 16):2635-42. doi: 10.1242/jcs.028647. Epub 2008 Jul 24. J Cell Sci. 2008. PMID: 18653540
-
Activation of Rac1 by RhoG regulates cell migration.J Cell Sci. 2006 Jan 1;119(Pt 1):56-65. doi: 10.1242/jcs.02720. Epub 2005 Dec 8. J Cell Sci. 2006. PMID: 16339170
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
-
The many faces of the guanine-nucleotide exchange factor trio.Cell Adh Migr. 2012 Nov-Dec;6(6):482-7. doi: 10.4161/cam.21418. Epub 2012 Oct 17. Cell Adh Migr. 2012. PMID: 23076143 Free PMC article. Review.
Cited by
-
Implications of Rho GTPase Signaling in Glioma Cell Invasion and Tumor Progression.Front Oncol. 2013 Oct 4;3:241. doi: 10.3389/fonc.2013.00241. Front Oncol. 2013. PMID: 24109588 Free PMC article. Review.
-
Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation.Front Physiol. 2014 Sep 30;5:374. doi: 10.3389/fphys.2014.00374. eCollection 2014. Front Physiol. 2014. PMID: 25324782 Free PMC article. Review.
-
Rho protein crosstalk: another social network?Trends Cell Biol. 2011 Dec;21(12):718-26. doi: 10.1016/j.tcb.2011.08.002. Epub 2011 Sep 15. Trends Cell Biol. 2011. PMID: 21924908 Free PMC article. Review.
-
Cellular Regulation of Macropinocytosis.Int J Mol Sci. 2024 Jun 26;25(13):6963. doi: 10.3390/ijms25136963. Int J Mol Sci. 2024. PMID: 39000072 Free PMC article. Review.
-
Syndecan-4 independently regulates multiple small GTPases to promote fibroblast migration during wound healing.Small GTPases. 2012 Apr-Jun;3(2):73-9. doi: 10.4161/sgtp.19301. Small GTPases. 2012. PMID: 22790193 Free PMC article.
References
-
- Araki N., Egami Y., Watanabe Y., Hatae T. Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp. Cell Res. 2007;313:1496–1507. - PubMed
-
- Beier I., Dusing R., Vetter H., Schmitz U. Epidermal growth factor stimulates Rac1 and p21-activated kinase in vascular smooth muscle cells. Atherosclerosis. 2008;196:92–97. - PubMed
-
- Bertrand-Duchesne M. P., Grenier D., Gagnon G. Epidermal growth factor released from platelet-rich plasma promotes endothelial cell proliferation in vitro. J. Periodontal Res. 2010;45:87–93. - PubMed
-
- Blangy A., Vignal E., Schmidt S., Debant A., Gauthier-Rouviere C., Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J. Cell Sci. 2000;113:729–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

