Multiplexed serologic assay for nine anogenital human papillomavirus types
- PMID: 20237197
- PMCID: PMC2863382
- DOI: 10.1128/CVI.00348-09
Multiplexed serologic assay for nine anogenital human papillomavirus types
Abstract
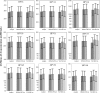
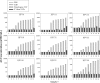
A multiplexed human papillomavirus (HPV) immunoassay has been developed for the detection of human IgG antibodies to HPV type 6, 11, 16, 18, 31, 33, 45, 52, and 58 virus-like particle (VLP) types in serum following natural infection or immunization with VLP-based vaccines. The VLP antigens were covalently conjugated to carboxyl Luminex microspheres (MS) using a carbodiimide chemistry. Antibody (Ab) titers were determined in a direct binding format, in which an IgG1- to -4-specific, phycoerythrin (PE)-labeled monoclonal antibody (MAb) (HP6043) binds to human serum IgG antibodies. Pooled serum samples from rhesus macaques immunized with a 9-valent VLP-based vaccine served as the reference standard. The overall specificity of the assay was >99%, and the linearity (parallelism) of the assay was <7% per 10-fold dilution. Total assay precision was <19% across 3 different VLP-microsphere lots, 2 secondary antibody lots, and 2 different operators over a period of 3 weeks. Three different methods were used to evaluate serostatus cutoffs (SCO): (i) a clinical sensitivity/specificity analysis based on "likely negative" and "likely positive" samples from nonvaccinees, (ii) stringent upper tolerance limits on samples from "likely negatives," and (iii) stringent upper tolerance limits from the same "likely negative" sample set after VLP adsorption. Depending on the method to set the serostatus cutoff, the percentage of seropositive samples at the month 48 time point following vaccination with the HPV 6/11/16/18 quadrivalent vaccine ranged from 70% to 100%. This assay has proven useful for measuring the levels of serum antibody to the nine HPV VLPs following natural infection or administration of VLP-based vaccines.
Figures


Similar articles
-
Development of a human papillomavirus competitive luminex immunoassay for 9 HPV types.Hum Vaccin Immunother. 2014;10(8):2168-74. doi: 10.4161/hv.29205. Hum Vaccin Immunother. 2014. PMID: 25424920 Free PMC article. Review.
-
Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18.Clin Diagn Lab Immunol. 2005 Aug;12(8):959-69. doi: 10.1128/CDLI.12.8.959-969.2005. Clin Diagn Lab Immunol. 2005. PMID: 16085914 Free PMC article.
-
International collaborative proficiency study of Human Papillomavirus type 16 serology.Vaccine. 2012 Jan 5;30(2):294-9. doi: 10.1016/j.vaccine.2011.10.096. Epub 2011 Nov 8. Vaccine. 2012. PMID: 22079074
-
The humoral response to Gardasil over four years as defined by total IgG and competitive Luminex immunoassay.Hum Vaccin. 2011 Feb;7(2):230-8. doi: 10.4161/hv.7.2.13948. Epub 2011 Feb 1. Hum Vaccin. 2011. PMID: 21307649
-
Chapter 12: Prophylactic HPV vaccines: underlying mechanisms.Vaccine. 2006 Aug 31;24 Suppl 3:S3/106-13. doi: 10.1016/j.vaccine.2006.05.110. Epub 2006 Jun 23. Vaccine. 2006. PMID: 16949996 Review.
Cited by
-
Seroprevalence of 8 oncogenic human papillomavirus genotypes and acquired immunity against reinfection.J Infect Dis. 2014 Aug 1;210(3):448-55. doi: 10.1093/infdis/jiu104. Epub 2014 Feb 25. J Infect Dis. 2014. PMID: 24569064 Free PMC article.
-
Development and validation of a multiplex immunoassay for the simultaneous quantification of type-specific IgG antibodies to E6/E7 oncoproteins of HPV16 and HPV18.PLoS One. 2020 Mar 26;15(3):e0229672. doi: 10.1371/journal.pone.0229672. eCollection 2020. PLoS One. 2020. PMID: 32214362 Free PMC article.
-
Evaluation of the Long-Term Anti-Human Papillomavirus 6 (HPV6), 11, 16, and 18 Immune Responses Generated by the Quadrivalent HPV Vaccine.Clin Vaccine Immunol. 2015 Aug;22(8):943-8. doi: 10.1128/CVI.00133-15. Epub 2015 Jun 17. Clin Vaccine Immunol. 2015. PMID: 26084514 Free PMC article. Clinical Trial.
-
Immunogenicity of 2 and 3 Doses of the Quadrivalent Human Papillomavirus Vaccine up to 120 Months Postvaccination: Follow-up of a Randomized Clinical Trial.Clin Infect Dis. 2020 Aug 14;71(4):1022-1029. doi: 10.1093/cid/ciz887. Clin Infect Dis. 2020. PMID: 31617568 Free PMC article. Clinical Trial.
-
Assessing the reduction of viral infectivity in HPV16/18-positive women after one, two, and three doses of Gardasil-9 (RIFT): Study protocol.PLoS One. 2024 May 20;19(5):e0304080. doi: 10.1371/journal.pone.0304080. eCollection 2024. PLoS One. 2024. PMID: 38768231 Free PMC article.
References
-
- Brady, J. F. 2006. Mathematical aspects of immunoassays, p. 249-270. In J. M. Van Emon (ed.), Immunoassay and other bioanalytical techniques. CRC Press, Boca Raton, FL.
-
- Buck, C. B., D. V. Pastrana, D. R. Lowy, and J. T. Schiller. 2005. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 119:445-462. - PubMed
-
- Capen, R., M. L. Shank-Retzlaff, H. Sings, M. Esser, C. Sattler, M. Washabaugh, and R. Sitrin. 2007. Establishing potency specifications for antigen vaccines. BioProcess Int. 5:30-43.
-
- Carter, J. J., L. A. Koutsky, J. P. Hughes, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 2000. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 181:1911-1919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

