Consequences of interference of milk with chemoattractants for enzyme-linked immunosorbent assay quantifications
- PMID: 20237202
- PMCID: PMC2863396
- DOI: 10.1128/CVI.00447-09
Consequences of interference of milk with chemoattractants for enzyme-linked immunosorbent assay quantifications
Abstract
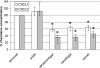
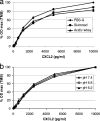
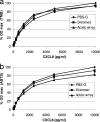
Concentrations of the chemoattractants CXCL1, CXCL2, CXCL3, CXCL8, and C5a in milk were reduced by the preparation of milk whey by high-speed centrifugation or with rennet. About half of the chemoattractants (35 to 65%) were associated with the casein micelle sediment, except when whey was prepared by acidification. Consequently, quantification of chemoattractants should be carried out preferentially with skimmed milk samples or, whenever whey is needed, with acidic whey samples. The interference of milk or milk whey with the enzyme-linked immunosorbent assays (ELISAs) used to quantify the chemoattractants was moderate, as long as tetramethylbenzidine (TMB), not ABTS [2,2'-azino-bis-(3-ethylbenzthiazoline-sulfonate)], was used as the substrate of peroxidase. These considerations will help to assess more precisely a component of the immune response of the mammary gland to infection.
Figures

Similar articles
-
Technical note: Direct enzyme immunoassay of progesterone in bovine milk whey.J Dairy Sci. 2005 Dec;88(12):4239-42. doi: 10.3168/jds.S0022-0302(05)73110-6. J Dairy Sci. 2005. PMID: 16291615
-
The chemokine CXCL3 is responsible for the constitutive chemotactic activity of bovine milk for neutrophils.Mol Immunol. 2008 Sep;45(15):4020-7. doi: 10.1016/j.molimm.2008.06.010. Epub 2008 Jul 26. Mol Immunol. 2008. PMID: 18657861
-
Effect of beta-lactoglobulin A and B whey protein variants on the rennet-induced gelation of skim milk gels in a model reconstituted skim milk system.J Dairy Sci. 2007 Feb;90(2):582-93. doi: 10.3168/jds.S0022-0302(07)71541-2. J Dairy Sci. 2007. PMID: 17235134
-
Staphylococcus aureus lipoteichoic acid triggers inflammation in the lactating bovine mammary gland.Vet Res. 2008 Sep-Oct;39(5):52. doi: 10.1051/vetres:2008034. Epub 2008 Jul 3. Vet Res. 2008. PMID: 18593548
-
Cellular prion protein in mammary gland and milk fractions of domestic ruminants.Biochem Biophys Res Commun. 2008 May 9;369(3):841-4. doi: 10.1016/j.bbrc.2008.02.108. Epub 2008 Mar 4. Biochem Biophys Res Commun. 2008. PMID: 18325321
Cited by
-
Innate and Adaptive Immunity Synergize to Trigger Inflammation in the Mammary Gland.PLoS One. 2016 Apr 21;11(4):e0154172. doi: 10.1371/journal.pone.0154172. eCollection 2016. PLoS One. 2016. PMID: 27100324 Free PMC article.
-
Antigen-Specific Mammary Inflammation Depends on the Production of IL-17A and IFN-γ by Bovine CD4+ T Lymphocytes.PLoS One. 2015 Sep 16;10(9):e0137755. doi: 10.1371/journal.pone.0137755. eCollection 2015. PLoS One. 2015. PMID: 26375594 Free PMC article.
-
Muramyl dipeptide synergizes with Staphylococcus aureus lipoteichoic acid to recruit neutrophils in the mammary gland and to stimulate mammary epithelial cells.Clin Vaccine Immunol. 2010 Nov;17(11):1797-809. doi: 10.1128/CVI.00268-10. Epub 2010 Sep 8. Clin Vaccine Immunol. 2010. PMID: 20826612 Free PMC article.
-
When is the best time to test paratuberculosis positivity? Observations from a follow-up study in Hungarian dairy herds.Front Vet Sci. 2025 Jun 19;12:1570915. doi: 10.3389/fvets.2025.1570915. eCollection 2025. Front Vet Sci. 2025. PMID: 40612149 Free PMC article.
-
T helper 17-associated cytokines are produced during antigen-specific inflammation in the mammary gland.PLoS One. 2013 May 16;8(5):e63471. doi: 10.1371/journal.pone.0063471. Print 2013. PLoS One. 2013. PMID: 23696826 Free PMC article.
References
-
- Bacon, K., M. Baggiolini, H. Broxmeyer, R. Horuk, I. Lindley, A. Mantovani, K. Maysushima, P. Murphy, H. Nomiyama, J. Oppenheim, A. Rot, T. Schall, M. Tsang, R. Thorpe, J. Van Damme, M. Wadhwa, O. Yoshie, A. Zlotnik, and K. Zoon. 2002. Chemokine/chemokine receptor nomenclature. J. Interferon Cytokine Res. 22:1067-1068. - PubMed
-
- Bannerman, D. D. 2009. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J. Anim. Sci. 87:10-25. - PubMed
-
- Bannerman, D. D., M. J. Paape, J. P. Goff, K. Kimura, J. D. Lippolis, and J. C. Hope. 2004. Innate immune response to intramammary infection with Serratia marcescens and Streptococcus uberis. Vet. Res. 35:681-700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

