The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges
- PMID: 20237240
- PMCID: PMC3365847
- DOI: 10.1210/er.2009-0023
The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges
Abstract
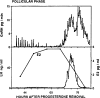
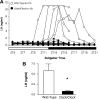
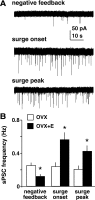
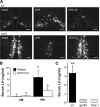
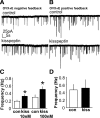
Ovarian steroids normally exert homeostatic negative feedback on GnRH release. During sustained exposure to elevated estradiol in the late follicular phase of the reproductive cycle, however, the feedback action of estradiol switches to positive, inducing a surge of GnRH release from the brain, which signals the pituitary LH surge that triggers ovulation. In rodents, this switch appears dependent on a circadian signal that times the surge to a specific time of day (e.g., late afternoon in nocturnal species). Although the precise nature of this daily signal and the mechanism of the switch from negative to positive feedback have remained elusive, work in the past decade has provided much insight into the role of circadian/diurnal and estradiol-dependent signals in GnRH/LH surge regulation and timing. Here we review the current knowledge of the neurobiology of the GnRH surge, in particular the actions of estradiol on GnRH neurons and their synaptic afferents, the regulation of GnRH neurons by fast synaptic transmission mediated by the neurotransmitters gamma-aminobutyric acid and glutamate, and the host of excitatory and inhibitory neuromodulators including kisspeptin, vasoactive intestinal polypeptide, catecholamines, neurokinin B, and RFamide-related peptides, that appear essential for GnRH surge regulation, and ultimately ovulation and fertility.
Figures
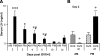
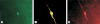
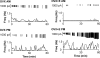



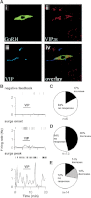


References
-
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E 1978 Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202:631–633 - PubMed
-
- Clarke IJ, Thomas GB, Yao B, Cummins JT 1987 GnRH secretion throughout the ovine estrous cycle. Neuroendocrinology 46:82–88 - PubMed
-
- Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E 1981 Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 - PubMed
-
- Marshall JC, Griffin ML 1993 The role of changing pulse frequency in the regulation of ovulation. Hum Reprod 8:57–61 - PubMed
-
- Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA 1991 A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology 128:509–517 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

