Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons
- PMID: 20237262
- PMCID: PMC2855130
- DOI: 10.1523/JNEUROSCI.6256-09.2010
Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons
Abstract
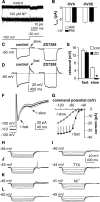
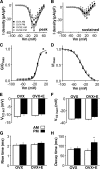
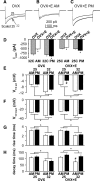
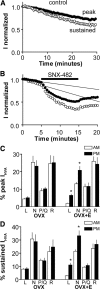
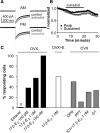
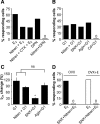
A robust surge of gonadotropin-releasing hormone (GnRH) release triggers the luteinizing hormone surge that induces ovulation. The GnRH surge is attributable to estradiol feedback, but the mechanisms are incompletely understood. Voltage-gated calcium channels (VGCCs) regulate hormone release and neuronal excitability, and may be part of the surge-generating mechanism. We examined VGCCs of GnRH neurons in brain slices from a model exhibiting daily luteinizing hormone surges. Mice were ovariectomized (OVX), and a subset was treated with estradiol implants (OVX+E). OVX+E mice exhibit negative feedback in the A.M. and positive feedback in the P.M. GnRH neurons express prominent high-voltage-activated (HVA) and small low-voltage-activated (LVA) macroscopic (whole-cell) Ca currents (I(Ca)). LVA-mediated currents were not altered by estradiol or time of day. In contrast, in OVX+E mice, HVA-mediated currents varied with time of day; HVA currents in cells from OVX+E mice were lower than those in cells from OVX mice in the A.M. but were higher in the P.M. These changes were attributable to diurnal alternations in L- and N-type components. There were no diurnal changes in any aspect of HVA-mediated I(Ca) in OVX mice. Acute in vitro treatment of cells from OVX and OVX+E mice with estradiol rapidly increased HVA currents primarily through L- and R-type VGCCs by activating estrogen receptor beta and GPR30, respectively. These results suggest multiple mechanisms contribute to the overall feedback regulation of HVA-mediated I(Ca) by estradiol. In combination with changes in synaptic inputs to GnRH neurons, these intrinsic changes in GnRH neurons may play critical roles in estradiol feedback.
Figures

Similar articles
-
Voltage-gated potassium currents are targets of diurnal changes in estradiol feedback regulation and kisspeptin action on gonadotropin-releasing hormone neurons in mice.Biol Reprod. 2011 Nov;85(5):987-95. doi: 10.1095/biolreprod.111.093492. Epub 2011 Jul 20. Biol Reprod. 2011. PMID: 21778142 Free PMC article.
-
Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice.Biol Reprod. 2009 Jun;80(6):1128-35. doi: 10.1095/biolreprod.108.075077. Epub 2009 Jan 28. Biol Reprod. 2009. PMID: 19176881 Free PMC article.
-
Progesterone treatment inhibits and dihydrotestosterone (DHT) treatment potentiates voltage-gated calcium currents in gonadotropin-releasing hormone (GnRH) neurons.Endocrinology. 2010 Nov;151(11):5349-58. doi: 10.1210/en.2010-0385. Epub 2010 Aug 25. Endocrinology. 2010. PMID: 20739401 Free PMC article.
-
The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges.Endocr Rev. 2010 Aug;31(4):544-77. doi: 10.1210/er.2009-0023. Epub 2010 Mar 17. Endocr Rev. 2010. PMID: 20237240 Free PMC article. Review.
-
Steroid regulation of GnRH neurons.Ann N Y Acad Sci. 2003 Dec;1007:143-52. doi: 10.1196/annals.1286.014. Ann N Y Acad Sci. 2003. PMID: 14993048 Review.
Cited by
-
Membrane-initiated actions of estradiol that regulate reproduction, energy balance and body temperature.Front Neuroendocrinol. 2012 Oct;33(4):376-87. doi: 10.1016/j.yfrne.2012.07.002. Epub 2012 Aug 1. Front Neuroendocrinol. 2012. PMID: 22871514 Free PMC article. Review.
-
Changes in GABAergic Transmission to and Intrinsic Excitability of Gonadotropin-Releasing Hormone (GnRH) Neurons during the Estrous Cycle in Mice.eNeuro. 2018 Nov 8;5(5):ENEURO.0171-18.2018. doi: 10.1523/ENEURO.0171-18.2018. eCollection 2018 Sep-Oct. eNeuro. 2018. PMID: 30417076 Free PMC article.
-
Proestrus Differentially Regulates Expression of Ion Channel and Calcium Homeostasis Genes in GnRH Neurons of Mice.Front Mol Neurosci. 2019 May 31;12:137. doi: 10.3389/fnmol.2019.00137. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31213979 Free PMC article.
-
Identified GnRH neuron electrophysiology: a decade of study.Brain Res. 2010 Dec 10;1364:10-24. doi: 10.1016/j.brainres.2010.09.066. Epub 2010 Nov 1. Brain Res. 2010. PMID: 20920482 Free PMC article. Review.
-
Regulation of endogenous conductances in GnRH neurons by estrogens.Brain Res. 2010 Dec 10;1364:25-34. doi: 10.1016/j.brainres.2010.08.096. Epub 2010 Sep 25. Brain Res. 2010. PMID: 20816765 Free PMC article. Review.
References
-
- Acerbo P, Nobile M. Temperature dependence of multiple high voltage activated Ca2+ channels in chick sensory neurones. Eur Biophys J. 1994;23:189–195. - PubMed
-
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
