RET signaling is required for survival and normal function of nonpeptidergic nociceptors
- PMID: 20237269
- PMCID: PMC2850282
- DOI: 10.1523/JNEUROSCI.5930-09.2010
RET signaling is required for survival and normal function of nonpeptidergic nociceptors
Abstract
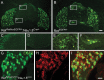
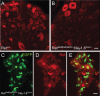
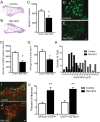
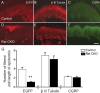
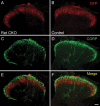
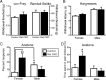
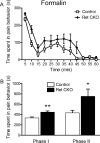
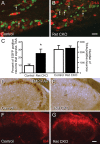
Small unmyelinated sensory neurons classified as nociceptors are divided into two subpopulations based on phenotypic differences, including expression of neurotrophic factor receptors. Approximately half of unmyelinated nociceptors express the NGF receptor TrkA, and half express the GDNF family ligand (GFL) receptor Ret. The function of NGF/TrkA signaling in the TrkA population of nociceptors has been extensively studied, and NGF/TrkA signaling is a well established mediator of pain. The GFLs are analgesic in models of neuropathic pain emphasizing the importance of understanding the physiological function of GFL/Ret signaling in nociceptors. However, perinatal lethality of Ret-null mice has precluded the study of the physiological role of GFL/Ret signaling in the survival, maintenance, and function of nociceptors in viable mice. We deleted Ret exclusively in nociceptors by crossing nociceptor-specific Na(v)1.8 Cre and Ret conditional mice to produce Ret-Na(v)1.8 conditional knock-out (CKO) mice. Loss of Ret exclusively in nociceptors results in a reduction in nociceptor number and size, indicating that Ret signaling is important for the survival and trophic support of these cells. Ret-Na(v)1.8 CKO mice exhibit reduced epidermal innervation but normal central projections. In addition, Ret-Na(v)1.8 CKO mice have increased sensitivity to cold and increased formalin-induced pain, demonstrating that Ret signaling modulates the function of nociceptors in vivo. Enhanced inflammation-induced pain may be mediated by decreased prostatic acid phosphatase (PAP), as PAP levels are markedly reduced in Ret-Na(v)1.8 CKO mice. The results of this study identify the physiological role of endogenous Ret signaling in the survival and function of nociceptors.
Figures
Similar articles
-
TNF-α/TNFR1 signaling is required for the development and function of primary nociceptors.Neuron. 2014 May 7;82(3):587-602. doi: 10.1016/j.neuron.2014.04.009. Neuron. 2014. PMID: 24811380 Free PMC article.
-
Essential role of Ret for defining non-peptidergic nociceptor phenotypes and functions in the adult mouse.Eur J Neurosci. 2011 Apr;33(8):1385-400. doi: 10.1111/j.1460-9568.2011.07634.x. Epub 2011 Mar 14. Eur J Neurosci. 2011. PMID: 21395865
-
A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons.Neuron. 2007 Jun 7;54(5):739-54. doi: 10.1016/j.neuron.2007.04.027. Neuron. 2007. PMID: 17553423
-
Glial cell line-derived neurotrophic factors (GFLs) and small molecules targeting RET receptor for the treatment of pain and Parkinson's disease.Cell Tissue Res. 2020 Oct;382(1):147-160. doi: 10.1007/s00441-020-03227-4. Epub 2020 Jun 17. Cell Tissue Res. 2020. PMID: 32556722 Free PMC article. Review.
-
Neurotrophic Factors and Nociceptor Sensitization.In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 2. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 2. PMID: 21882462 Free Books & Documents. Review.
Cited by
-
Natural Selection Equally Supports the Human Tendencies in Subordination and Domination: A Genome-Wide Study With in silico Confirmation and in vivo Validation in Mice.Front Genet. 2019 Feb 20;10:73. doi: 10.3389/fgene.2019.00073. eCollection 2019. Front Genet. 2019. PMID: 30873204 Free PMC article.
-
Myelinating Schwann cells ensheath multiple axons in the absence of E3 ligase component Fbxw7.Nat Commun. 2019 Jul 5;10(1):2976. doi: 10.1038/s41467-019-10881-y. Nat Commun. 2019. PMID: 31278268 Free PMC article.
-
The development of somatosensory neurons: Insights into pain and itch.Curr Top Dev Biol. 2021;142:443-475. doi: 10.1016/bs.ctdb.2020.10.005. Epub 2020 Nov 5. Curr Top Dev Biol. 2021. PMID: 33706924 Free PMC article. Review.
-
Ion channels and pain in Fabry disease.Mol Pain. 2021 Jan-Dec;17:17448069211033172. doi: 10.1177/17448069211033172. Mol Pain. 2021. PMID: 34284652 Free PMC article. Review.
-
Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance.Nat Neurosci. 2014 Oct;17(10):1351-61. doi: 10.1038/nn.3809. Epub 2014 Sep 7. Nat Neurosci. 2014. PMID: 25195104 Free PMC article.
References
-
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. - PubMed
-
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. - PubMed
-
- Akkina SK, Patterson CL, Wright DE. GDNF rescues nonpeptidergic unmyelinated primary afferents in streptozotocin-treated diabetic mice. Exp Neurol. 2001;167:173–182. - PubMed
-
- Bennett DL, Koltzenburg M, Priestley JV, Shelton DL, McMahon SB. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur J Neurosci. 1998a;10:1282–1291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK081644/DK/NIDDK NIH HHS/United States
- P30 NS057105/NS/NINDS NIH HHS/United States
- R01 DK082531/DK/NIDDK NIH HHS/United States
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R21NS059566/NS/NINDS NIH HHS/United States
- R01 NS042595/NS/NINDS NIH HHS/United States
- P30-DK079333/DK/NIDDK NIH HHS/United States
- G9717869/MRC_/Medical Research Council/United Kingdom
- R01 DK081644/DK/NIDDK NIH HHS/United States
- R21 NS059566/NS/NINDS NIH HHS/United States
- P30 DK079333/DK/NIDDK NIH HHS/United States
- R01 AG013730/AG/NIA NIH HHS/United States
- DK082531/DK/NIDDK NIH HHS/United States
- K08 HD047396/HD/NICHD NIH HHS/United States
- HD047396/HD/NICHD NIH HHS/United States
- AGO13730/PHS HHS/United States
- NS042595/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
