Removal of polysialic acid triggers dispersion of subventricularly derived neuroblasts into surrounding CNS tissues
- PMID: 20237270
- PMCID: PMC6632263
- DOI: 10.1523/JNEUROSCI.4382-09.2010
Removal of polysialic acid triggers dispersion of subventricularly derived neuroblasts into surrounding CNS tissues
Abstract
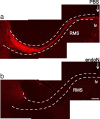
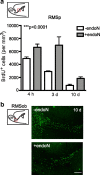
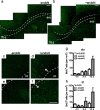
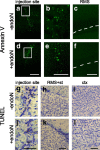
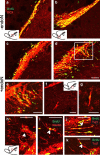
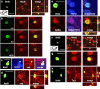
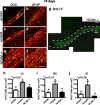
Cells generated in the subventricular zone give rise to neuroblasts that migrate to the olfactory bulb (OB) along the rostral migratory stream (RMS). The polysialylated form of neural cell adhesion molecule (PSA-NCAM) is expressed by these cells, and has been shown to both promote their migration and suppress differentiation induced by NCAM. In the present study, enzymatic removal of PSA from these neuroblasts using PSA-specific endoneuraminidase has been found not only to disrupt the tangential migration and cellular organization of the RMS, but also to cause a massive dispersion of BrdU (5-bromo-2'-deoxyuridine)-labeled neuroblasts into surrounding brain regions, including cortex and striatum. These dispersed cells are capable of differentiation, some into mature neurons, and could potentially be of value in the repair of CNS injury. Although the removal of PSA by genetic deletion of NCAM also results in a smaller OB and a swollen RMS, the cells do not escape the RMS in large numbers. These findings suggest that the presence of NCAM without PSA plays a role in the dispersion process, possibly by inducing a new pattern of migration associated with NCAM-dependent differentiation.
Figures


References
-
- Alonso G, Prieto M, Chauvet N. Tangential migration of young neurons arising from the subventricular zone of adult rats is impaired by surgical lesions passing through their natural migratory pathway. J Comp Neurol. 1999;405:508–528. - PubMed
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
