Pals1 is a major regulator of the epithelial-like polarization and the extension of the myelin sheath in peripheral nerves
- PMID: 20237282
- PMCID: PMC6632289
- DOI: 10.1523/JNEUROSCI.5185-09.2010
Pals1 is a major regulator of the epithelial-like polarization and the extension of the myelin sheath in peripheral nerves
Abstract
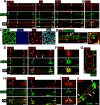
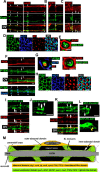
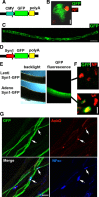
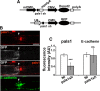
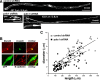
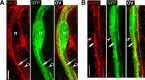
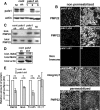
Diameter, organization, and length of the myelin sheath are important determinants of the nerve conduction velocity, but the basic molecular mechanisms that control these parameters are only partially understood. Cell polarization is an essential feature of differentiated cells, and relies on a set of evolutionarily conserved cell polarity proteins. We investigated the molecular nature of myelin sheath polarization in connection with the functional role of the cell polarity protein pals1 (Protein Associated with Lin Seven 1) during peripheral nerve myelin sheath extension. We found that, in regard to epithelial polarity, the Schwann cell outer abaxonal domain represents a basolateral-like domain, while the inner adaxonal domain and Schmidt-Lanterman incisures form an apical-like domain. Silencing of pals1 in myelinating Schwann cells in vivo resulted in a severe reduction of myelin sheath thickness and length. Except for some infoldings, the structure of compact myelin was not fundamentally affected, but cells produced less myelin turns. In addition, pals1 is required for the normal polarized localization of the vesicular markers sec8 and syntaxin4, and for the distribution of E-cadherin and myelin proteins PMP22 and MAG at the plasma membrane. Our data show that the polarity protein pals1 plays an essential role in the radial and longitudinal extension of the myelin sheath, likely involving a functional role in membrane protein trafficking. We conclude that regulation of epithelial-like polarization is a critical determinant of myelin sheath structure and function.
Figures


References
-
- Anitei M, Ifrim M, Ewart MA, Cowan AE, Carson JH, Bansal R, Pfeiffer SE. A role for Sec8 in oligodendrocyte morphological differentiation. J Cell Sci. 2006;119:807–818. - PubMed
-
- Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. - PubMed
-
- Chan JR, Jolicoeur C, Yamauchi J, Elliott J, Fawcett JP, Ng BK, Cayouette M. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
