Localized suppression of cortical growth hormone-releasing hormone receptors state-specifically attenuates electroencephalographic delta waves
- PMID: 20237285
- PMCID: PMC2846621
- DOI: 10.1523/JNEUROSCI.6047-09.2010
Localized suppression of cortical growth hormone-releasing hormone receptors state-specifically attenuates electroencephalographic delta waves
Abstract
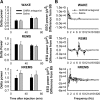
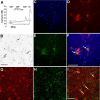
Growth hormone-releasing hormone (GHRH) promotes non-rapid eye movement sleep (NREMS), in part via a well characterized hypothalamic sleep-promoting site. However, GHRH may also act in the cortex to influence sleep. Application of GHRH to the surface of the cortex changes electroencephalographic (EEG) delta power. GHRH and the GHRH receptor (GHRHR) mRNAs are detectable in the rat cortex, and the expression of cortical GHRHR is activity dependent. Here, we microinjected a GHRH antagonist or GHRHR small interfering RNA (siGHRHR) onto the somatosensory cortex surface in rats. The unilateral application of the GHRH antagonist ipsilaterally decreased EEG delta wave power during NREMS, but not wakefulness, during the initial 40 min after injection. Similarly, the injection of siGHRHR reduced cortical expression of GHRHR and suppressed NREMS EEG delta wave power during 20-24 h after injection. Using the fura-2 calcium imaging technique, cultured cortical cells responded to GHRH by increasing intracellular calcium. Approximately 18% of the GHRH-responsive cells were GABAergic as illustrated by glutamic acid decarboxylase-67 (GAD67) immunostaining. Double labeling for GAD67 and GHRHR in vitro and in vivo indicated that only a minority of cortical GHRHR-containing cells were GABAergic. Our data suggest that endogenous cortical GHRH activates local cortical cells to affect EEG delta wave power state-specifically. Results are also consistent with the hypothesis that GHRH contributes to local network state regulation.
Figures



Similar articles
-
Growth hormone-releasing hormone: cerebral cortical sleep-related EEG actions and expression.Am J Physiol Regul Integr Comp Physiol. 2007 Aug;293(2):R922-30. doi: 10.1152/ajpregu.00237.2007. Epub 2007 May 30. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 17537840
-
Sleep in spontaneous dwarf rats.Brain Res. 2006 Sep 7;1108(1):133-46. doi: 10.1016/j.brainres.2006.06.016. Epub 2006 Jul 21. Brain Res. 2006. PMID: 16859658
-
Role of growth hormone-releasing hormone in sleep and growth impairments induced by upper airway obstruction in rats.Eur Respir J. 2011 Oct;38(4):870-7. doi: 10.1183/09031936.00197610. Epub 2011 Mar 15. Eur Respir J. 2011. PMID: 21406516
-
Regulation of the pituitary somatotroph cell by GHRH and its receptor.Recent Prog Horm Res. 2000;55:237-66; discussion 266-7. Recent Prog Horm Res. 2000. PMID: 11036940 Review.
-
Growth Hormone Releasing Hormone (GHRH) and the GHRH Receptor.Rev Endocr Metab Disord. 2002 Dec;3(4):313-23. doi: 10.1023/a:1020949507265. Rev Endocr Metab Disord. 2002. PMID: 12424433 Review. No abstract available.
Cited by
-
Growth hormone releasing hormone (GHRH) signaling modulates intermittent hypoxia-induced oxidative stress and cognitive deficits in mouse.J Neurochem. 2013 Nov;127(4):531-40. doi: 10.1111/jnc.12360. Epub 2013 Jul 19. J Neurochem. 2013. PMID: 23815362 Free PMC article.
-
Knockdown of orexin type 2 receptor in the lateral pontomesencephalic tegmentum of rats increases REM sleep.Eur J Neurosci. 2013 Mar;37(6):957-63. doi: 10.1111/ejn.12101. Epub 2013 Jan 3. Eur J Neurosci. 2013. PMID: 23282008 Free PMC article.
-
TRANSLATION OF BRAIN ACTIVITY INTO SLEEP.Hirosaki Igaku. 2012;63(Suppl):S1-S16. Hirosaki Igaku. 2012. PMID: 24795496 Free PMC article.
-
Control of sleep and wakefulness.Physiol Rev. 2012 Jul;92(3):1087-187. doi: 10.1152/physrev.00032.2011. Physiol Rev. 2012. PMID: 22811426 Free PMC article. Review.
-
Time- and behavioral state-dependent changes in posterior hypothalamic GABAA receptors contribute to the regulation of sleep.PLoS One. 2014 Jan 21;9(1):e86545. doi: 10.1371/journal.pone.0086545. eCollection 2014. PLoS One. 2014. PMID: 24466145 Free PMC article.
References
-
- Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;609:284–292. - PubMed
-
- Boisvert C, Paré C, Veyrat-Durebex C, Robert A, Dubuisson S, Morel G, Gaudreau P. Localization and regulation of a functional GHRH receptor in the rat renal medulla. Endocrinology. 2002;143:1475–1484. - PubMed
-
- Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. - PubMed
-
- Bredow S, Taishi P, Obál F, Jr, Guha-Thakurta N, Krueger JM. Hypothalamic growth hormone-releasing hormone mRNA varies across the day in rats. Neuroreport. 1996;7:2501–2505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
