Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors
- PMID: 20237287
- PMCID: PMC5390116
- DOI: 10.1523/JNEUROSCI.5806-09.2010
Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors
Abstract
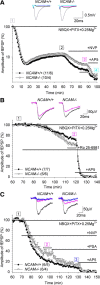
The neural cell adhesion molecule (NCAM) is the predominant carrier of alpha2,8 polysialic acid (PSA) in the mammalian brain. Abnormalities in PSA and NCAM expression are associated with schizophrenia in humans and cause deficits in hippocampal synaptic plasticity and contextual fear conditioning in mice. Here, we show that PSA inhibits opening of recombinant NMDA receptors composed of GluN1/2B (NR1/NR2B) or GluN1/2A/2B (NR1/NR2A/NR2B) but not of GluN1/2A (NR1/NR2A) subunits. Deficits in NCAM/PSA increase GluN2B-mediated transmission and Ca(2+) transients in the CA1 region of the hippocampus. In line with elevation of GluN2B-mediated transmission, defects in long-term potentiation in the CA1 region and contextual fear memory in NCAM/PSA-deficient mice are abrogated by application of a GluN2B-selective antagonist. Furthermore, treatment with the glutamate scavenger glutamic-pyruvic transaminase, ablation of Ras-GRF1 (a mediator of GluN2B signaling to p38 MAPK), or direct inhibition of hyperactive p38 MAPK can restore impaired synaptic plasticity in brain slices lacking PSA/NCAM. Thus, PSA carried by NCAM regulates plasticity and learning by inhibition of the GluN2B-Ras-GRF1-p38 MAPK signaling pathway. These findings implicate carbohydrates carried by adhesion molecules in modulating NMDA receptor signaling in the brain and demonstrate reversibility of cognitive deficits associated with ablation of a schizophrenia-related adhesion molecule.
Figures





Similar articles
-
Polysialylated neural cell adhesion molecule is involved in induction of long-term potentiation and memory acquisition and consolidation in a fear-conditioning paradigm.J Neurosci. 2006 Oct 18;26(42):10888-109898. doi: 10.1523/JNEUROSCI.0878-06.2006. J Neurosci. 2006. PMID: 17050727 Free PMC article.
-
Restoration of synaptic plasticity and learning in young and aged NCAM-deficient mice by enhancing neurotransmission mediated by GluN2A-containing NMDA receptors.J Neurosci. 2012 Feb 15;32(7):2263-75. doi: 10.1523/JNEUROSCI.5103-11.2012. J Neurosci. 2012. PMID: 22396402 Free PMC article.
-
Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GRF2 in the induction of long-term potentiation and long-term depression.J Neurosci. 2006 Feb 8;26(6):1721-9. doi: 10.1523/JNEUROSCI.3990-05.2006. J Neurosci. 2006. PMID: 16467520 Free PMC article.
-
Regulation of extrasynaptic signaling by polysialylated NCAM: Impact for synaptic plasticity and cognitive functions.Mol Cell Neurosci. 2017 Jun;81:12-21. doi: 10.1016/j.mcn.2016.11.005. Epub 2016 Nov 16. Mol Cell Neurosci. 2017. PMID: 27865768 Review.
-
The neural cell adhesion molecule in synaptic plasticity and ageing.Int J Dev Neurosci. 2000 Apr-Jun;18(2-3):193-9. doi: 10.1016/s0736-5748(99)00088-x. Int J Dev Neurosci. 2000. PMID: 10715574 Review.
Cited by
-
Glutamate Attenuates the Survival Property of IGFR through NR2B Containing N-Methyl-D-aspartate Receptors in Cortical Neurons.Oxid Med Cell Longev. 2020 Aug 11;2020:5173184. doi: 10.1155/2020/5173184. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32849999 Free PMC article.
-
Biological roles of glycans.Glycobiology. 2017 Jan;27(1):3-49. doi: 10.1093/glycob/cww086. Epub 2016 Aug 24. Glycobiology. 2017. PMID: 27558841 Free PMC article. Review.
-
Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration.Physiol Rev. 2014 Apr;94(2):461-518. doi: 10.1152/physrev.00033.2013. Physiol Rev. 2014. PMID: 24692354 Free PMC article. Review.
-
Decreased Serum NCAM Levels Associated with Cognitive Impairment in Vascular Dementia.Dis Markers. 2021 Aug 31;2021:2792884. doi: 10.1155/2021/2792884. eCollection 2021. Dis Markers. 2021. PMID: 34504627 Free PMC article.
-
Distinctive PSA-NCAM and NCAM hallmarks in glutamate-induced dendritic atrophy and synaptic disassembly.PLoS One. 2014 Oct 3;9(10):e108921. doi: 10.1371/journal.pone.0108921. eCollection 2014. PLoS One. 2014. PMID: 25279838 Free PMC article.
References
-
- Angata K, Chan D, Thibault J, Fukuda M. Molecular dissection of the ST8Sia IV polysialyltransferase. Distinct domains are required for neural cell adhesion molecule recognition and polysialylation. J Biol Chem. 2004;279:25883–25890. - PubMed
-
- Arai M, Yamada K, Toyota T, Obata N, Haga S, Yoshida Y, Nakamura K, Minabe Y, Ujike H, Sora I, Ikeda K, Mori N, Yoshikawa T, Itokawa M. Association between polymorphisms in the promoter region of the sialyltransferase 8B (SIAT8B) gene and schizophrenia. Biol Psychiatry. 2006;59:652–659. - PubMed
-
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. - PubMed
-
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
