Cystine 186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption
- PMID: 20237315
- PMCID: PMC2879102
- DOI: 10.1182/blood-2009-09-241356
Cystine 186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption
Abstract
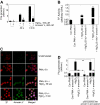
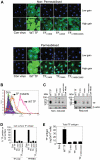
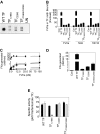
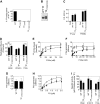
Tissue factor (TF) on cell surfaces resides mostly in a cryptic state. It is not entirely clear how cryptic TF differs from procoagulantly active TF and how deencryption occurs. Here, we critically evaluated the importance of cystine 186-cystine 209 (Cys186-Cys209) bond formation for TF procoagulant activity and its de-encryption. Chinese hamster ovary cells transfected with TF(C186S), TF(C209S), or TF(C186S/C209S) expressed little procoagulant activity at the cell surface. TF monoclonal antibody and activated factor VII (FVIIa) binding studies showed that little TF protein was present at the cell surface in cells expressing mutant TF. Similar data were obtained in human umbilical vein endothelial cells (HUVECs) transduced to express TF(C186S), TF(C209S), or TF(C186S/C209S). Analysis of TF activity in HUVECs expressing similar levels of wild-type TF and TF(C186S/C209S) showed that TF mutant in the presence of saturating concentrations of FVIIa exhibited similar coagulant activity as that of wild-type TF. More importantly, treatment of HUVECs expressing TF(C186S/C209S) with HgCl(2) or ionomycin increased the cell-surface TF activity to the same extent as that of the wild-type TF. Our data provide clear evidence that TF lacking the Cys186-Cys209 bond is coagulantly active once it is complexed with FVIIa, and TF de-encryption does not require Cys186-Cys209 disulfide bond formation.
Figures


Comment in
-
Tissue factor mutated at the allosteric Cys186-Cys209 disulfide bond is severely impaired in decrypted procoagulant activity.Blood. 2010 Jul 22;116(3):500-1; author reply 502-3. doi: 10.1182/blood-2010-04-281287. Blood. 2010. PMID: 20651086 Free PMC article. No abstract available.
-
Complete abolishment of coagulant activity in monomeric disulfide-deficient tissue factor.Blood. 2011 Sep 22;118(12):3446-8. doi: 10.1182/blood-2011-06-364612. Blood. 2011. PMID: 21940832 No abstract available.
Similar articles
-
Tissue factor mutated at the allosteric Cys186-Cys209 disulfide bond is severely impaired in decrypted procoagulant activity.Blood. 2010 Jul 22;116(3):500-1; author reply 502-3. doi: 10.1182/blood-2010-04-281287. Blood. 2010. PMID: 20651086 Free PMC article. No abstract available.
-
Contribution of allosteric disulfide in the structural regulation of membrane-bound tissue factor-factor VIIa binary complex.J Biomol Struct Dyn. 2019 Sep;37(14):3707-3720. doi: 10.1080/07391102.2018.1526118. Epub 2018 Nov 13. J Biomol Struct Dyn. 2019. PMID: 30238846
-
Tissue factor encryption and decryption: facts and controversies.Thromb Res. 2012 May;129 Suppl 2(Suppl 2):S13-7. doi: 10.1016/j.thromres.2012.02.021. Epub 2012 Mar 6. Thromb Res. 2012. PMID: 22398016 Free PMC article. Review.
-
Evidence for activation of tissue factor by an allosteric disulfide bond.Biochemistry. 2006 Oct 3;45(39):12020-8. doi: 10.1021/bi061271a. Biochemistry. 2006. PMID: 17002301
-
Tissue factor de-encryption, thrombus formation, and thiol-disulfide exchange.Semin Thromb Hemost. 2013 Feb;39(1):40-7. doi: 10.1055/s-0032-1333311. Epub 2013 Jan 16. Semin Thromb Hemost. 2013. PMID: 23325480 Review.
Cited by
-
Regulation of tissue factor coagulant activity on cell surfaces.J Thromb Haemost. 2012 Nov;10(11):2242-53. doi: 10.1111/jth.12003. J Thromb Haemost. 2012. PMID: 23006890 Free PMC article. Review.
-
Tissue factor mutated at the allosteric Cys186-Cys209 disulfide bond is severely impaired in decrypted procoagulant activity.Blood. 2010 Jul 22;116(3):500-1; author reply 502-3. doi: 10.1182/blood-2010-04-281287. Blood. 2010. PMID: 20651086 Free PMC article. No abstract available.
-
Tissue factor: mechanisms of decryption.Front Biosci (Elite Ed). 2012 Jan 1;4(4):1513-27. doi: 10.2741/477. Front Biosci (Elite Ed). 2012. PMID: 22201972 Free PMC article. Review.
-
Disulfide reduction abolishes tissue factor cofactor function.Biochim Biophys Acta. 2013 Jun;1830(6):3489-96. doi: 10.1016/j.bbagen.2013.02.013. Epub 2013 Feb 20. Biochim Biophys Acta. 2013. PMID: 23434438 Free PMC article.
-
Increasing the sensitivity of the human microvesicle tissue factor activity assay.Thromb Res. 2019 Oct;182:64-74. doi: 10.1016/j.thromres.2019.07.011. Epub 2019 Jul 19. Thromb Res. 2019. PMID: 31450010 Free PMC article.
References
-
- Rapaport SI, Rao LVM. The tissue factor pathway: how it has become a “prima ballerina.”. Thromb Haemost. 1995;74(1):7–17. - PubMed
-
- Konigsberg W, Kirchhofer D, Riederer MA, Nemerson Y. The TF:VIIa complex: clinical significance, structure-function relationships and its role in signaling and metastasis. Thromb Haemost. 2001;86(3):757–771. - PubMed
-
- Taubman MB, Fallon JT, Schecter AD, et al. Tissue factor in the pathogenesis of atherosclerosis. Thromb Haemost. 1997;78(1):200–204. - PubMed
-
- Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005;25(8):1545–1550. - PubMed
-
- Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24(6):1015–1022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

