CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency
- PMID: 20237408
- PMCID: PMC2846042
- DOI: 10.1172/JCI39748
CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency
Abstract
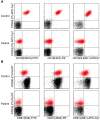
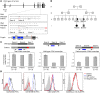
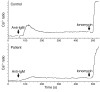
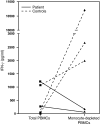
Antibody deficiencies constitute the largest group of symptomatic primary immunodeficiency diseases. In several patients, mutations in CD19 have been found to underlie disease, demonstrating the critical role for the protein encoded by this gene in antibody responses; CD19 functions in a complex with CD21, CD81, and CD225 to signal with the B cell receptor upon antigen recognition. We report here a patient with severe nephropathy and profound hypogammaglobulinemia. The immunodeficiency was characterized by decreased memory B cell numbers, impaired specific antibody responses, and an absence of CD19 expression on B cells. The patient had normal CD19 alleles but carried a homozygous CD81 mutation resulting in a complete lack of CD81 expression on blood leukocytes. Retroviral transduction and glycosylation experiments on EBV-transformed B cells from the patient revealed that CD19 membrane expression critically depended on CD81. Similar to CD19-deficient patients, CD81-deficient patients had B cells that showed impaired activation upon stimulation via the B cell antigen receptor but no overt T cell subset or function defects. In this study, we present what we believe to be the first antibody deficiency syndrome caused by a mutation in the CD81 gene and consequent disruption of the CD19 complex on B cells. These findings may contribute to unraveling the genetic basis of antibody deficiency syndromes and the nonredundant functions of CD81 in humans.
Figures


References
-
- Pan-Hammarstrom Q, Hammarstrom L. Antibody deficiency diseases. Eur J Immunol. 2008;38(2):327–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

