Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model
- PMID: 20237412
- PMCID: PMC2846036
- DOI: 10.1172/JCI37223
Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model
Erratum in
- J Clin Invest. 2010 Nov 1;120(11):4163
Abstract
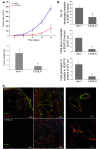
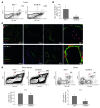
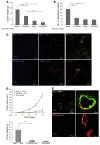
Angiogenesis is a hallmark of malignant neoplasias, as the formation of new blood vessels is required for tumors to acquire oxygen and nutrients essential for their continued growth and metastasis. However, the signaling pathways leading to tumor vascularization are not fully understood. Here, using a transplantable mouse tumor model, we have demonstrated that endogenous IFN-beta inhibits tumor angiogenesis through repression of genes encoding proangiogenic and homing factors in tumor-infiltrating neutrophils. We determined that IFN-beta-deficient mice injected with B16F10 melanoma or MCA205 fibrosarcoma cells developed faster-growing tumors with better-developed blood vessels than did syngeneic control mice. These tumors displayed enhanced infiltration by CD11b+Gr1+ neutrophils expressing elevated levels of the genes encoding the proangiogenic factors VEGF and MMP9 and the homing receptor CXCR4. They also expressed higher levels of the transcription factors c-myc and STAT3, known regulators of VEGF, MMP9, and CXCR4. In vitro, treatment of these tumor-infiltrating neutrophils with low levels of IFN-beta restored expression of proangiogenic factors to control levels. Moreover, depletion of these neutrophils inhibited tumor growth in both control and IFN-beta-deficient mice. We therefore suggest that constitutively produced endogenous IFN-beta is an important mediator of innate tumor surveillance. Further, we believe our data help to explain the therapeutic effect of IFN treatment during the early stages of cancer development.
Figures

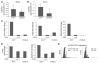


References
-
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82(1):4–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

