Chronicles of a death foretold: dual sequential cell death checkpoints in TNF signaling
- PMID: 20237426
- PMCID: PMC4114106
- DOI: 10.4161/cc.9.6.10982
Chronicles of a death foretold: dual sequential cell death checkpoints in TNF signaling
Abstract
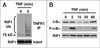
The kinase RIP1 wears a coat of many colors during TNF receptor signaling and can regulate both activation of pro-survival NFkB and programmed cell death pathways. In this review, we outline how coating RIP1 with K63-linked ubiquitin chains forms a protective layer that prevents RIP1 from binding apoptotic regulators and serves as an early guard against cell death. Further on, binding of NFkB signaling components to the ubiquitin coat of RIP1 activates long-term pro-survival signaling and forms a more impenetrable suit of armor against cell death. If RIP1 is not decorated with ubiquitin chains it becomes an unstoppable harbinger of bad news: programmed cell death.
Figures
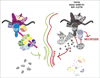
Similar articles
-
Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination.Cell Death Dis. 2015 Jun 25;6(6):e1800. doi: 10.1038/cddis.2015.158. Cell Death Dis. 2015. PMID: 26111062 Free PMC article.
-
Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling.Curr Biol. 2007 Mar 6;17(5):418-24. doi: 10.1016/j.cub.2007.01.027. Epub 2007 Feb 15. Curr Biol. 2007. PMID: 17306544 Free PMC article.
-
Diverse ubiquitin linkages regulate RIP kinases-mediated inflammatory and cell death signaling.Cell Death Differ. 2017 Jul;24(7):1160-1171. doi: 10.1038/cdd.2017.33. Epub 2017 May 5. Cell Death Differ. 2017. PMID: 28475174 Free PMC article. Review.
-
Coordinated ubiquitination and phosphorylation of RIP1 regulates necroptotic cell death.Cell Death Differ. 2017 Jan;24(1):26-37. doi: 10.1038/cdd.2016.78. Epub 2016 Aug 12. Cell Death Differ. 2017. PMID: 27518435 Free PMC article.
-
The role of the kinases RIP1 and RIP3 in TNF-induced necrosis.Sci Signal. 2010 Mar 30;3(115):re4. doi: 10.1126/scisignal.3115re4. Sci Signal. 2010. PMID: 20354226 Review.
Cited by
-
The role of TRADD in death receptor signaling.Cell Cycle. 2012 Mar 1;11(5):871-6. doi: 10.4161/cc.11.5.19300. Epub 2012 Mar 1. Cell Cycle. 2012. PMID: 22333735 Free PMC article. Review.
-
Ripoptocide - A Spark for Inflammation.Front Cell Dev Biol. 2019 Aug 13;7:163. doi: 10.3389/fcell.2019.00163. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31457011 Free PMC article. Review.
-
Immune dysregulation in SHARPIN-deficient mice is dependent on CYLD-mediated cell death.Proc Natl Acad Sci U S A. 2021 Dec 14;118(50):e2001602118. doi: 10.1073/pnas.2001602118. Proc Natl Acad Sci U S A. 2021. PMID: 34887354 Free PMC article.
-
Zfra is a small wizard in the mitochondrial apoptosis.Aging (Albany NY). 2010 Dec;2(12):1023-9. doi: 10.18632/aging.100263. Aging (Albany NY). 2010. PMID: 21212468 Free PMC article.
-
CYLD Proteolysis Protects Macrophages from TNF-Mediated Auto-necroptosis Induced by LPS and Licensed by Type I IFN.Cell Rep. 2016 Jun 14;15(11):2449-61. doi: 10.1016/j.celrep.2016.05.032. Epub 2016 Jun 2. Cell Rep. 2016. PMID: 27264187 Free PMC article.
References
-
- Natoli G, Costanzo A, Guido F, Moretti F, Levrero M. Apoptotic, non-apoptotic and anti-apoptotic pathways of tumor necrosis factor signalling. Biochem Pharmacol. 1998;56:915–920. - PubMed
-
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NFkappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. - PubMed
-
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. - PubMed
-
- Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NFkappaB activation prevents cell death. Cell. 1996;87:565–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
