Acute stress impairs hippocampal mossy fiber-CA3 long-term potentiation by enhancing cAMP-specific phosphodiesterase 4 activity
- PMID: 20237461
- PMCID: PMC3055459
- DOI: 10.1038/npp.2010.33
Acute stress impairs hippocampal mossy fiber-CA3 long-term potentiation by enhancing cAMP-specific phosphodiesterase 4 activity
Abstract
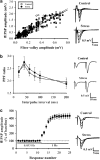
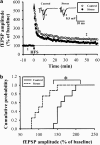
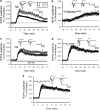
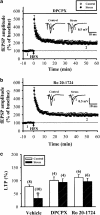
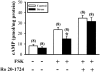
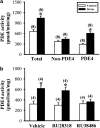
The mossy fiber synapses onto hippocampal CA3 neurons show unique molecular features and a wide dynamic range of plasticity. Although acute stress has been well recognized to alter bidirectional long-term synaptic plasticity in the hippocampal CA1 region and dentate gyrus, it remains unclear whether the same effect may also occur at the mossy fiber-CA3 synapses. Here, we report that hippocampal slices prepared from adult mice that had experienced an acute unpredictable and inescapable restraint tail-shock stress showed a marked impairment of long-term potentiation (LTP) induced by high-frequency stimulation or adenylyl cyclase activator forskolin. This effect was prevented when animals were submitted to bilateral adrenalectomy or given the glucocorticoid receptor antagonist RU38486 before experiencing stress. In contrast, stress has no effect on synaptic potentiation induced by the non-hydrolysable and membrane-permeable cyclic adenosine 5'-monophosphate (cAMP) analog Sp-8-bromo-cAMPS. No obvious differences were observed between control and stressed mice in the basal synaptic transmission, paired-pulse facilitation, or frequency facilitation at the mossy fiber-CA3 synapses. We also found that the inhibitory effect of stress on mossy fiber LTP was obviated by the adenosine A(1) receptor antagonist 8-cyclopentyl-1,3,-dipropylxanthine, the non-specific phosphodiesterase (PDE) inhibitor 3-isobutyl-methylxanthine, and the specific PDE4 inhibitor 4-(3-butoxy-4-methoxyphenyl)methyl-2-imidazolidone. In addition, stress induces a sustained and profound increase in cAMP-specific PDE4 activity. These results suggest that the inhibition of mossy fiber LTP by acute stress treatment seems originating from a corticosterone-induced sustained increase in the PDE4 activity to accelerate the metabolism of cAMP to adenosine, in turn triggering an adenosine A(1) receptor-mediated impairment of transmitter release machinery.
Figures

References
-
- Alzheimer C, Röhrenbeck J, ten Bruggencate G. Adenosine depresses induction of LTP at the mossy fiber-CA3 synapse in vitro. Brain Res. 1991;543:163–165. - PubMed
-
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci. 1999;2:720–726. - PubMed
-
- Bruns RF, Lu GH, Pugsley TA. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986;29:331–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

