Human primary gastric dendritic cells induce a Th1 response to H. pylori
- PMID: 20237463
- PMCID: PMC3683837
- DOI: 10.1038/mi.2010.10
Human primary gastric dendritic cells induce a Th1 response to H. pylori
Abstract
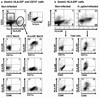
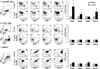
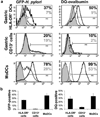
Adaptive CD4 T-cell responses are important in the pathogenesis of chronic Helicobacter pylori gastritis. However, the gastric antigen-presenting cells that induce these responses have not yet been identified. Here we show that dendritic cells (DCs) are present in the gastric mucosa of healthy subjects and are more prevalent and more activated in the gastric mucosa of H. pylori-infected subjects. H. pylori induced gastric DCs isolated from noninfected subjects to express increased levels of CD11c, CD86 and CD83, and to secrete proinflammatory cytokines, particularly interleukin (IL)-6 and IL-8. Importantly, gastric DCs pulsed with live H. pylori, but not control DCs, mediated T-cell secretion of interferon-gamma. The ability of H. pylori to induce gastric DC maturation and stimulate gastric DC activation of Th1 cells implicates gastric DCs as initiators of the immune response to H. pylori.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures



References
-
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer. 2006;118:3030–3044. - PubMed
-
- Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer. 2002;2:28–37. - PubMed
-
- Bamford KB, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. - PubMed
-
- Kim JM, Kim JS, Jung HC, Song IS, Kim CY. Up-regulation of inducible nitric oxide synthase and nitric oxide in Helicobacter pylori -infected human gastric epithelial cells: possible role of interferon-gamma in polarized nitric oxide secretion. Helicobacter. 2002;7:116–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI083539/AI/NIAID NIH HHS/United States
- DK-47322/DK/NIDDK NIH HHS/United States
- R24 DK064400/DK/NIDDK NIH HHS/United States
- AI-78217/AI/NIAID NIH HHS/United States
- RR-20136/RR/NCRR NIH HHS/United States
- C06 RR020136/RR/NCRR NIH HHS/United States
- R01 DK054495/DK/NIDDK NIH HHS/United States
- AI-83539/AI/NIAID NIH HHS/United States
- R01 DK084063/DK/NIDDK NIH HHS/United States
- R01 DK047322/DK/NIDDK NIH HHS/United States
- DK-64400/DK/NIDDK NIH HHS/United States
- DK-084063/DK/NIDDK NIH HHS/United States
- I01 CX000372/CX/CSRD VA/United States
- DK-54495/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

