IL-12 can alleviate Th17-mediated allergic lung inflammation through induction of pulmonary IL-10 expression
- PMID: 20237464
- PMCID: PMC3816527
- DOI: 10.1038/mi.2010.9
IL-12 can alleviate Th17-mediated allergic lung inflammation through induction of pulmonary IL-10 expression
Abstract
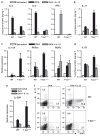
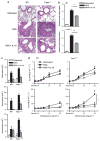
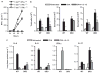
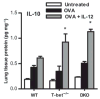
Interleukin (IL)-12 has been shown to suppress T helper type 2 (Th2)-induced pathogenesis that is associated with allergic asthma, largely through interferon (IFN)-gamma production. We have recently shown that in the absence of T-bet, the major regulator of IFN-gamma expression, allergic lung inflammation is primarily associated with IL-17-associated recruitment of neutrophils into the pulmonary tract of mice. In the absence of T-bet, exogenous IL-12 was still able to suppress neutrophilic infiltration and to diminish levels of IL-17, IL-23, and IL-23R, as well as retinoic acid-related orphan receptor gamma t, the transcriptional regulator of the Th17 pathway. The same effects were observed in T-bet(-/-) IFN-gamma(-/-) double knockout mice, showing an IFN-gamma-independent effect of IL-12 in this model. IL-10 expression in the lungs of T-bet-deficient mice was significantly increased after IL-12 treatment, and inoculation of anti-IL-10R mAb completely reversed the ability of IL-12 to suppress histological inflammation, recruitment of inflammatory cell subsets into the lung, bronchiole hyperresponsiveness, and IL-17 production. We conclude that Th17-mediated allergic lung inflammation that becomes dominant in the absence of effective IFN-gamma signaling can be effectively suppressed by IL-12 through an IL-10-dependent mechanism.
Conflict of interest statement
The author declared no conflict of interest.
Figures
Similar articles
-
IL-12 contributes to allergen-induced airway inflammation in experimental asthma.J Immunol. 2006 Nov 1;177(9):6460-70. doi: 10.4049/jimmunol.177.9.6460. J Immunol. 2006. PMID: 17056578
-
[Effects of fermented white mustard seed plaster on airway immune balance and its inflammatory response mechanism in bronchial asthma rats].Zhen Ci Yan Jiu. 2025 Mar 25;50(3):287-294. doi: 10.13702/j.1000-0607.20230975. Zhen Ci Yan Jiu. 2025. PMID: 40103380 Chinese.
-
IL-12-STAT4-IFN-gamma axis is a key downstream pathway in the development of IL-13-mediated asthma phenotypes in a Th2 type asthma model.Exp Mol Med. 2010 Aug 31;42(8):533-46. doi: 10.3858/emm.2010.42.8.054. Exp Mol Med. 2010. PMID: 20592486 Free PMC article.
-
Regulation of interleukin-10 and interleukin-22 expression in T helper cells.Curr Opin Immunol. 2011 Oct;23(5):605-12. doi: 10.1016/j.coi.2011.07.018. Epub 2011 Aug 20. Curr Opin Immunol. 2011. PMID: 21862302 Review.
-
T cells in asthma: influences of genetics, environment, and T-cell plasticity.J Allergy Clin Immunol. 2013 May;131(5):1267-74; quiz 1275. doi: 10.1016/j.jaci.2013.02.016. Epub 2013 Mar 28. J Allergy Clin Immunol. 2013. PMID: 23541326 Review.
Cited by
-
Differing effects of interleukin-10 on cutaneous and pulmonary Francisella tularensis live vaccine strain infection.Infect Immun. 2013 Jun;81(6):2022-7. doi: 10.1128/IAI.00024-13. Epub 2013 Mar 25. Infect Immun. 2013. PMID: 23529615 Free PMC article.
-
Lung CD103+ dendritic cells restrain allergic airway inflammation through IL-12 production.JCI Insight. 2017 May 18;2(10):e90420. doi: 10.1172/jci.insight.90420. eCollection 2017 May 18. JCI Insight. 2017. PMID: 28515363 Free PMC article.
-
High-dose dexamethasone induced LPS-stimulated rat alveolar macrophages apoptosis.Drug Des Devel Ther. 2017 Oct 25;11:3097-3104. doi: 10.2147/DDDT.S147014. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 29123381 Free PMC article.
-
Role of IL-10-producing regulatory B cells in modulating T-helper cell immune responses during silica-induced lung inflammation and fibrosis.Sci Rep. 2016 Jun 29;6:28911. doi: 10.1038/srep28911. Sci Rep. 2016. PMID: 27354007 Free PMC article.
-
Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses.Cell Host Microbe. 2012 Jul 19;12(1):34-46. doi: 10.1016/j.chom.2012.05.017. Cell Host Microbe. 2012. PMID: 22817986 Free PMC article.
References
-
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. - PubMed
-
- Nakajima H, Takatsu K. Role of cytokines in allergic airway inflammation. Int Arch Allergy Immunol. 2007;142:265–273. - PubMed
-
- Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–412. - PubMed
-
- Jatakanon A, et al. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160:1532–1539. - PubMed
-
- Louis R, et al. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161:9–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

