Sodium channelopathies of skeletal muscle result from gain or loss of function
- PMID: 20237798
- PMCID: PMC2883924
- DOI: 10.1007/s00424-010-0814-4
Sodium channelopathies of skeletal muscle result from gain or loss of function
Abstract
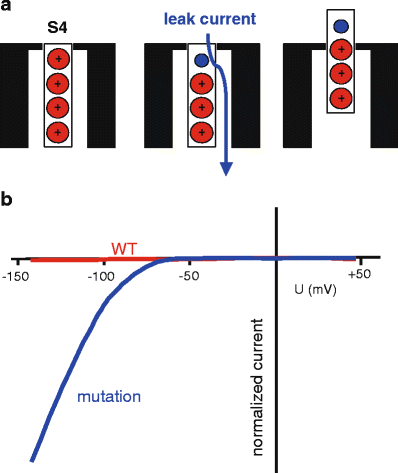
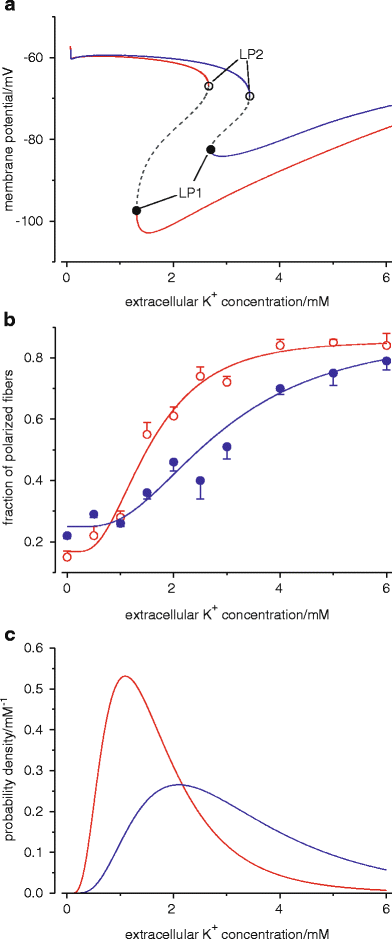
Five hereditary sodium channelopathies of skeletal muscle have been identified. Prominent symptoms are either myotonia or weakness caused by an increase or decrease of muscle fiber excitability. The voltage-gated sodium channel NaV1.4, initiator of the muscle action potential, is mutated in all five disorders. Pathogenetically, both loss and gain of function mutations have been described, the latter being the more frequent mechanism and involving not just the ion-conducting pore, but aberrant pores as well. The type of channel malfunction is decisive for therapy which consists either of exerting a direct effect on the sodium channel, i.e., by blocking the pore, or of restoring skeletal muscle membrane potential to reduce the fraction of inactivated channels.
Figures

Similar articles
-
Cold-induced defects of sodium channel gating in atypical periodic paralysis plus myotonia.Neurology. 2008 Mar 4;70(10):755-61. doi: 10.1212/01.wnl.0000265397.70057.d8. Epub 2007 Sep 26. Neurology. 2008. PMID: 17898326 Free PMC article.
-
Prevalence study of genetically defined skeletal muscle channelopathies in England.Neurology. 2013 Apr 16;80(16):1472-5. doi: 10.1212/WNL.0b013e31828cf8d0. Epub 2013 Mar 20. Neurology. 2013. PMID: 23516313 Free PMC article.
-
Slow inactivation: slow but not dull.Neurology. 2008 Mar 4;70(10):746-7. doi: 10.1212/01.wnl.0000304253.88642.8d. Neurology. 2008. PMID: 18316687 No abstract available.
-
Novel insights into the pathomechanisms of skeletal muscle channelopathies.Curr Neurol Neurosci Rep. 2012 Feb;12(1):62-9. doi: 10.1007/s11910-011-0238-3. Curr Neurol Neurosci Rep. 2012. PMID: 22083238 Review.
-
Therapy in myotonic disorders and in muscle channelopathies.Neurol Sci. 2000;21(5 Suppl):S953-61. doi: 10.1007/s100720070009. Neurol Sci. 2000. PMID: 11382195 Review.
Cited by
-
Characterization of the honeybee AmNaV1 channel and tools to assess the toxicity of insecticides.Sci Rep. 2015 Jul 23;5:12475. doi: 10.1038/srep12475. Sci Rep. 2015. PMID: 26202396 Free PMC article.
-
Evidence for Non-neutral Evolution in a Sodium Channel Gene in African Weakly Electric Fish (Campylomormyrus, Mormyridae).J Mol Evol. 2016 Aug;83(1-2):61-77. doi: 10.1007/s00239-016-9754-8. Epub 2016 Aug 1. J Mol Evol. 2016. PMID: 27481396
-
Sodium channels, the electrogenisome and the electrogenistat: lessons and questions from the clinic.J Physiol. 2012 Jun 1;590(11):2601-12. doi: 10.1113/jphysiol.2012.228460. Epub 2012 Mar 12. J Physiol. 2012. PMID: 22411010 Free PMC article. Review.
-
Location: A surrogate for personalized treatment of sodium channelopathies.Ann Neurol. 2018 Jul;84(1):1-9. doi: 10.1002/ana.25268. Ann Neurol. 2018. PMID: 30048009 Free PMC article.
-
Myotonia congenita: mutation spectrum of CLCN1 in Spanish patients.J Genet. 2019 Sep;98:71. J Genet. 2019. PMID: 31544778
References
-
- Bouhours M, Sternberg D, Davoine CS, Ferrer X, Willer JC, Fontaine B, Tabti N. Functional characterization and cold sensitivity of T1313A, a new mutation of the skeletal muscle sodium channel causing paramyotonia congenita in humans. J Physiol. 2004;554:635–647. doi: 10.1113/jphysiol.2003.053082. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

