Nanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-infected macrophages: implications for neuroAIDS therapeutics
- PMID: 20237859
- PMCID: PMC3401515
- DOI: 10.1007/s11481-010-9198-7
Nanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-infected macrophages: implications for neuroAIDS therapeutics
Abstract
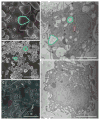
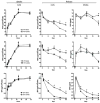
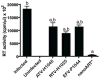
We posit that improvements in pharmacokinetics and biodistributions of antiretroviral therapies (ART) for human immunodeficiency virus type one-infected people can be achieved through nanoformulationed drug delivery systems. To this end, we manufactured nanoparticles of atazanavir, efavirenz, and ritonavir (termed nanoART) and treated human monocyte-derived macrophages (MDM) in combination therapies to assess antiretroviral responses. This resulted in improved drug uptake, release, and antiretroviral efficacy over monotherapy. MDM rapidly, within minutes, ingested nanoART combinations, at equal or similar rates, as individual formulations. Combination nanoART ingested by MDM facilitated individual drug release from 15 to >20 days. These findings are noteworthy as a nanoART cell-mediated drug delivery provides a means to deliver therapeutics to viral sanctuaries, such as the central nervous system during progressive human immunodeficiency virus type one infection. The work brings us yet another step closer to realizing the utility of nanoART for virus-infected people.
Figures


References
-
- Anttila S, Hukkanen J, Hakkola J, Stjernvall T, Beaune P, Edwards RJ, Boobis AR, Pelkonen O, Raunio H. Expression and localization of CYP3A4 and CYP3A5 in human lung. Am J Respir Cell Mol Biol. 1997;16:242–249. - PubMed
-
- Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50:93–99. - PubMed
-
- Bruce RD, Altice FL, Gourevitch MN, Friedland GH. Pharmacokinetic drug interactions between opioid agonist therapy and antiretroviral medications: implications and management for clinical practice. J Acquir Immune Defic Syndr. 2006a;41:563–572. - PubMed
-
- Bruce RD, McCance-Katz E, Kharasch ED, Moody DE, Morse GD. Pharmacokinetic interactions between buprenorphine and antiretroviral medications. Clin Infect Dis. 2006b;43(Suppl 4):S216–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- P01MH64570/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- P01 NS31492/NS/NINDS NIH HHS/United States
- R03 CA156357/CA/NCI NIH HHS/United States
- 2R01 NS034239/NS/NINDS NIH HHS/United States
- P20RR 15635/RR/NCRR NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R37 NS036126/NS/NINDS NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- P20 RR015635/RR/NCRR NIH HHS/United States
- 2R37 NS36126/NS/NINDS NIH HHS/United States
- P01 NS43985/NS/NINDS NIH HHS/United States
- R01 CA089225/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

