Hypoxia inducible factor-1α (HIF-1α) and some HIF-1 target genes are elevated in experimental glaucoma
- PMID: 20237864
- PMCID: PMC3612432
- DOI: 10.1007/s12031-010-9343-z
Hypoxia inducible factor-1α (HIF-1α) and some HIF-1 target genes are elevated in experimental glaucoma
Abstract
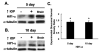
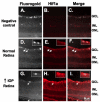
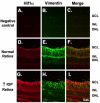
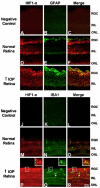
Low levels of hypoxia have been suggested to be a mechanism of retinal damage in glaucoma. To test the hypothesis that the activation of the hypoxia-responsive transcription factor hypoxia inducible factor-1alpha (HIF-1alpha) is involved in the pathophysiology of glaucoma, we used a rat model of glaucoma to study (1) HIF-1alpha retinal protein levels by immunoblot analysis, (2) cellular localization of HIF-1alpha in the retina by immunohistochemistry, and (3) expression of retinal HIF-1 gene targets by quantitative real-time polymerase chain reaction. Glaucoma was unilaterally induced in rats by injecting hypertonic saline in episcleral veins. We find that HIF-1alpha protein was increased in the retina following elevation of intraocular pressure, specifically in Müller glia and astrocytes but not in activated microglia. Eight established HIF-1 target genes were measured in experimental glaucoma. Retinal Epo, Flt-1, Hsp-27, Pai-1, and Vegfa mRNA levels were increased and Et-1, Igf2, and Tgfbeta3 levels were decreased in the glaucomatous retinas. Thus, the increase in HIF-1alpha levels in Müller glia and astrocytes is accompanied by a marked up regulation of some, but not all, HIF-1 transcriptional targets. These data support the hypothesis that HIF-1alpha becomes transcriptionally active in astrocytes and Müller cells but not microglia or neurons in glaucoma, arguing against a global hypoxia stimulus to the retina.
Figures
Similar articles
-
Role of hypoxia-inducible factor-1α in preconditioning-induced protection of retinal ganglion cells in glaucoma.Mol Vis. 2013 Nov 23;19:2360-72. eCollection 2013. Mol Vis. 2013. PMID: 24319330 Free PMC article.
-
Erythropoietin Protects Retinal Cells in Diabetic Rats Through Upregulating ZnT8 via Activating ERK Pathway and Inhibiting HIF-1α Expression.Invest Ophthalmol Vis Sci. 2015 Dec;56(13):8166-78. doi: 10.1167/iovs.15-18093. Invest Ophthalmol Vis Sci. 2015. PMID: 26720469
-
The expression of heat shock protein 27 in retinal ganglion and glial cells in a rat glaucoma model.Neuroscience. 2007 Dec 12;150(3):692-704. doi: 10.1016/j.neuroscience.2007.09.078. Epub 2007 Oct 11. Neuroscience. 2007. PMID: 17993247
-
Does elevated intraocular pressure reduce retinal TRKB-mediated survival signaling in experimental glaucoma?Exp Eye Res. 2009 Dec;89(6):921-33. doi: 10.1016/j.exer.2009.08.003. Epub 2009 Aug 14. Exp Eye Res. 2009. PMID: 19682984 Free PMC article.
-
Pharmacological regulation of HIF-1α, RGC death, and glaucoma.Curr Opin Pharmacol. 2024 Aug;77:102467. doi: 10.1016/j.coph.2024.102467. Epub 2024 Jun 18. Curr Opin Pharmacol. 2024. PMID: 38896924 Review.
Cited by
-
Topical Curcumin Nanocarriers are Neuroprotective in Eye Disease.Sci Rep. 2018 Jul 23;8(1):11066. doi: 10.1038/s41598-018-29393-8. Sci Rep. 2018. PMID: 30038334 Free PMC article.
-
Integrative transcriptomic and proteomic analysis reveals CD9/ITGA4/PI3K-Akt axis mediates trabecular meshwork cell apoptosis in human glaucoma.J Cell Mol Med. 2020 Jan;24(1):814-829. doi: 10.1111/jcmm.14792. Epub 2019 Nov 3. J Cell Mol Med. 2020. PMID: 31680442 Free PMC article.
-
Ocular Hypertension Results in Hypoxia within Glia and Neurons throughout the Visual Projection.Antioxidants (Basel). 2022 Apr 29;11(5):888. doi: 10.3390/antiox11050888. Antioxidants (Basel). 2022. PMID: 35624752 Free PMC article.
-
Retinal Protection of New Nutraceutical Formulation.Pharmaceutics. 2025 Jan 7;17(1):73. doi: 10.3390/pharmaceutics17010073. Pharmaceutics. 2025. PMID: 39861721 Free PMC article.
-
Virus-mediated EpoR76E gene therapy preserves vision in a glaucoma model by modulating neuroinflammation and decreasing oxidative stress.J Neuroinflammation. 2016 Feb 15;13:39. doi: 10.1186/s12974-016-0499-5. J Neuroinflammation. 2016. PMID: 26876380 Free PMC article.
References
-
- Ahmed F, Brown KM, Stephan DA, Morrison JC, Johnson EC, Tomarev SI. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2004;45(4):1247–1258. - PubMed
-
- Benn SC, Perrelet D, Kato AC, et al. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36(1):45–56. - PubMed
-
- Bilton RL, Booker GW. The subtle side to hypoxia inducible factor (HIFalpha) regulation. Eur J Biochem. 2003;270(5):791–798. - PubMed
-
- Brahimi-Horn MC, Pouyssegur J. HIF at a glance. J Cell Sci. 2009;122(Pt 8):1055–1057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

