Oxidative stress and the ubiquitin proteolytic system in age-related macular degeneration
- PMID: 20238046
- PMCID: PMC5757835
- DOI: 10.1007/978-1-4419-1399-9_51
Oxidative stress and the ubiquitin proteolytic system in age-related macular degeneration
Abstract
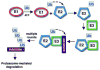
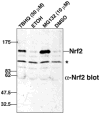
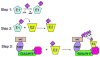
AMD is a leading cause of irreversible vision loss in people over 60 years of age. Although the pathogenesis of this disease is multifactorial, clinical studies have revealed that oxidative damage is a significant etiological factor. The ubiquitin proteolytic system (UPS) plays a major cytoprotective role in the retina. It accomplishes this largely by degrading oxidatively-damaged proteins to prevent their toxic accumulation. In this review, we discuss numerous features of the UPS in the retina and propose various ways that components of the UPS can be harnessed for therapeutic intervention in AMD. We discuss published work describing the distribution of various UPS enzymes in different retinal cell types and present new findings describing the localization of the class III ubiquitin conjugating enzymes. These enzymes are functional homologues of a pair of yeast enzymes that mediate the degradation of misfolded and oxidatively-damaged proteins. We also discuss recent work showing that only newly synthesized proteins which have incurred oxidative damage are targeted for degradation by the UPS whereas the turnover of oxidatively-damaged, long-lived proteins is largely unchanged. Additionally, we review recent work describing how polyubiquitylation influences the sorting of damaged proteins into one of two novel intracellular compartments. Finally, we discuss how the UPS modulates the stability and activity of Nrf2, the major anti-oxidant transcription factor in the retina.
Figures


Similar articles
-
Interplay between Autophagy and the Ubiquitin-Proteasome System and Its Role in the Pathogenesis of Age-Related Macular Degeneration.Int J Mol Sci. 2019 Jan 8;20(1):210. doi: 10.3390/ijms20010210. Int J Mol Sci. 2019. PMID: 30626110 Free PMC article. Review.
-
Expression and distribution of the class III ubiquitin-conjugating enzymes in the retina.Mol Vis. 2010 Nov 18;16:2425-37. Mol Vis. 2010. PMID: 21139979 Free PMC article.
-
Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics).Aging Cell. 2012 Feb;11(1):1-13. doi: 10.1111/j.1474-9726.2011.00752.x. Epub 2011 Nov 15. Aging Cell. 2012. PMID: 21967227 Free PMC article.
-
Oxidative Stress and the Nrf2 Anti-Oxidant Transcription Factor in Age-Related Macular Degeneration.Adv Exp Med Biol. 2016;854:67-72. doi: 10.1007/978-3-319-17121-0_10. Adv Exp Med Biol. 2016. PMID: 26427395 Free PMC article. Review.
-
Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD.Autophagy. 2014;10(11):1989-2005. doi: 10.4161/auto.36184. Autophagy. 2014. PMID: 25484094 Free PMC article.
Cited by
-
The Role of the Ubiquitin System in Eye Diseases.Life (Basel). 2025 Mar 20;15(3):504. doi: 10.3390/life15030504. Life (Basel). 2025. PMID: 40141848 Free PMC article. Review.
-
Comparative transcriptome analysis of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) reveals novel insights into heat stress tolerance in insects.BMC Genomics. 2017 Dec 19;18(1):974. doi: 10.1186/s12864-017-4355-5. BMC Genomics. 2017. PMID: 29258441 Free PMC article.
-
iPS cell modeling of Best disease: insights into the pathophysiology of an inherited macular degeneration.Hum Mol Genet. 2013 Feb 1;22(3):593-607. doi: 10.1093/hmg/dds469. Epub 2012 Nov 8. Hum Mol Genet. 2013. PMID: 23139242 Free PMC article.
-
Proteomic analysis of retinal pigment epithelium cells after exposure to UVA radiation.BMC Ophthalmol. 2019 Aug 2;19(1):168. doi: 10.1186/s12886-019-1151-9. BMC Ophthalmol. 2019. PMID: 31375076 Free PMC article.
-
Comparative Transcriptome Analysis of the Heat Stress Response in Monochamus alternatus Hope (Coleoptera: Cerambycidae).Front Physiol. 2020 Jan 21;10:1568. doi: 10.3389/fphys.2019.01568. eCollection 2019. Front Physiol. 2020. PMID: 32038275 Free PMC article.
References
-
- Benolken RM, Anderson RE, Wheeler TG. Membrane fatty acids associated with the electrical response in visual excitation. Science. 1973;182:1253–1254. ( https://doi.org/10.1126/science.182.4118.1253) - DOI - PubMed
-
- Bonfanti L, Candeo P, Piccinini M, et al. Distribution of protein gene product 9.5 (PGP 9.5) in the vertebrate retina: evidence that immunoreactivity is restricted to mammalian horizontal and ganglion cells. J Comp Neurol. 1992;322:35–44. ( https://doi.org/10.1002/cne.903220104) - DOI - PubMed
-
- Bosu DR, Kipreos ET. Cullin-RING ubiquitin ligases: global regulation and activation cycles. Cell Div. 2008;3:7. ( https://doi.org/10.1186/1747-1028-3-7) - DOI - PMC - PubMed
-
- Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. ( https://doi.org/10.1073/pnas.222551899) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

