Olanzapine versus other atypical antipsychotics for schizophrenia
- PMID: 20238348
- PMCID: PMC4169107
- DOI: 10.1002/14651858.CD006654.pub2
Olanzapine versus other atypical antipsychotics for schizophrenia
Abstract
Background: In many countries of the industrialised world second generation ("atypical") antipsychotics have become the first line drug treatment for people with schizophrenia. The question as to whether, and if so how much, the effects of the various second generation antipsychotics differ is a matter of debate. In this review we examined how the efficacy and tolerability of olanzapine differs from that of other second generation antipsychotics.
Objectives: To evaluate the effects of olanzapine compared to other atypical antipsychotics for people with schizophrenia and schizophrenia-like psychosis.
Search strategy: 1. Electronic searching We searched the Cochrane Schizophrenia Group Trials Register (April 2007) which is based on regular searches of BIOSIS, CENTRAL, CINAHL, EMBASE, MEDLINE and PsycINFO.2. Reference searching We inspected the reference of all identified studies for more trials.3. Personal contact We contacted the first author of each included study for missing information.4. Drug companies We contacted the manufacturers of all atypical antipsychotics included for additional data.
Selection criteria: We included all randomised trials that used at least single-blind (rater-blind) design, comparing oral olanzapine with oral forms of amisulpride, aripiprazole, clozapine, quetiapine, risperidone, sertindole, ziprasidone or zotepine in people with schizophrenia or schizophrenia-like psychosis.
Data collection and analysis: We extracted data independently. For dichotomous data we calculated relative risks (RR) and their 95% confidence intervals (CI) on an intention-to-treat basis based on a random effects model. We calculated numbers needed to treat/harm (NNT/NNH) where appropriate. For continuous data, we calculated weighted mean differences (WMD) again based on a random effects model.
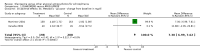
Main results: The review currently includes 50 studies and 9476 participants which provided data for six comparisons (olanzapine compared to amisulpride, aripiprazole, clozapine, quetiapine, risperidone or ziprasidone). The overall attrition from the included studies was considerable (49.2%) leaving the interpretation of results problematic.Olanzapine improved the general mental state (PANSS total score) more than aripiprazole (2 RCTs, n=794, WMD -4.96 CI -8.06 to -1.85), quetiapine (10 RCTs, n=1449, WMD -3.66 CI -5.39 to -1.93), risperidone (15 RCTs, n=2390, WMD -1.94 CI -3.31 to -0.58) and ziprasidone (4 RCTs, n=1291, WMD -8.32 CI -10.99 to -5.64), but not more than amisulpride or clozapine. This somewhat better efficacy was confirmed by fewer participants in the olanzapine groups leaving the studies early due to inefficacy of treatment compared to quetiapine (8 RCTs, n=1563, RR 0.56 CI 0.44 to 0.70, NNT 11 CI 6 to 50), risperidone (14 RCTs, n=2744, RR 0.78 CI 0.62 to 0.98, NNT 50 CI 17 to 100) and ziprasidone (5 RCTs, n=1937, RR 0.64 CI 0.51 to 0.79, NNT 17, CI 11 to 33).Fewer participants in the olanzapine group than in the quetiapine (2 RCTs, n=876, RR 0.56 CI 0.41 to 0.77, NNT 11 CI 7 to 25) and ziprasidone (2 RCTs, n=766, RR 0.65 CI 0.45 to 0.93, NNT 17 CI 9 to 100) treatment groups, but not in the clozapine group (1 RCT, n=980, RR 1.28 CI 1.02 to 1.61, NNH not estimable), had to be re-hospitalised in the trials.Except for clozapine, all comparators induced less weight gain than olanzapine (olanzapine compared to amisulpride: 3 RCTs, n=671, WMD 2.11kg CI 1.29kg to 2.94kg; aripiprazole: 1 RCT, n=90, WMD 5.60kg CI 2.15kg to 9.05kg; quetiapine: 7 RCTs, n=1173, WMD 2.68kg CI 1.10kg to 4.26kg; risperidone: 13 RCTs, n=2116, WMD 2.61kg CI 1.48kg to 3.74kg; ziprasidone: 5 RCTs, n=1659, WMD 3.82kg CI 2.96kg to 4.69kg). Associated problems such as glucose and cholesterol increase were usually also more frequent in the olanzapine group.Other differences in adverse effects were less well documented. Nevertheless, olanzapine may be associated with slightly more extrapyramidal side effects than quetiapine (use of antiparkinson medication (6 RCTs, n=1090, RR 2.05 CI 1.26 to 3.32, NNH 25 CI 14 to 100), but less than risperidone (use of antiparkinson medication 13 RCTs, n=2599, RR 0.78 CI 0.65 to 0.95, NNH 17 CI 9 to 100) and ziprasidone (use of antiparkinson medication 4 RCTs, n=1732, RR 0.70 CI 0.50 to 0.97, NNH not estimable). It may also increase prolactin somewhat more than aripiprazole, clozapine and quetiapine, but clearly less so than risperidone (6 RCTs, n=1291, WMD -22.84 CI -27.98 to -17.69).
Authors' conclusions: Olanzapine may be a somewhat more efficacious drug than some other second generation antipsychotic drugs. This small superiority in efficacy needs to be weighed against a larger weight gain and associated metabolic problems than most other second generation antipsychotic drugs, except clozapine. These conclusions are tentative due to the large number of people leaving the studies early which possibly limits the validity of the findings. Further large, well-designed trials are necessary to establish the relative effects of different second generation antipsychotic drugs.
Conflict of interest statement
Katja Komossa: none. Stefan Leucht received speaker/consultancy honoria from Sanofi‐Aventis, BMS, Eli Lilly, Janssen, Lundbeck and Pfizer. He received research support from Sanofi‐Aventis and Eli Lilly. Christine Rummel received lecture honoraria and travel grants to attend scientific meetings from AstraZeneca, Janssen‐Cilag, Eli Lilly and Pfizer. Werner Kissling: received speaker or consultancy honoraria from SanofiAventis, BMS, Lilly, Janssen, Lundbeck, Bayer and Pfizer. Heike Hunger: none. Franziska Schmidt: none Sandra Schwarz: none. Lorna Duggan: has attended functions sponsored by Lundbeck, Janssen, Pfizer, Bristol Myers Squibb and Zeneca and has accepted sponsorship from Eli Lilly for internal flights in the United States.
Figures
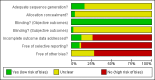
























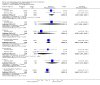























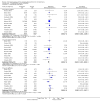




















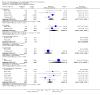











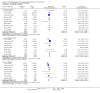































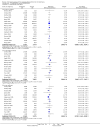
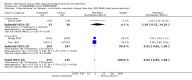













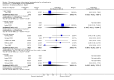


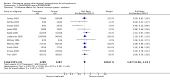

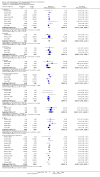





















































Update of
References
References to studies included in this review
Atmaca 2003 {published data only}
-
- Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Serum leptin and triglyceride levels in patients on treatment with atypical antipsychotics. Journal of Clinical Psychiatry 2003;64(5):598‐604. - PubMed
Bai 2005 {published data only}
-
- Bai YM, Ping LY, Lin CC, Wang YC, Liou YJ, Wu BJ, Chen TT, Chen JY, Lin CY, Chou P. Comparative effects of atypical antipsychotic on tardive dyskinesia and neurocognition: a 24‐week randomized, single‐blind, controlled study. European College of Neuropsychopharmacology 2005;15(Suppl 3):S473.
Bitter 2004 {published data only}
-
- Bitter I, Dossenbach MRK, Brook S, Feldman PD, Metcalfe S, Gagiano CA, Furedi J, Bartko G, Janka Z, Banki CM, Kovacs G, Breier A. Olanzapine versus clozapine in treatment‐resistant or treatment‐intolerant schizophrenia. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2004;28:173‐80. - PubMed
Breier 2005 {published data only}
-
- Breier A, Berg PH, Thakore JH, Naber D, Gattaz WF, Cavazzoni P, Walker DJ, Roychowdhury SM, Kane JM. Olanzapine versus ziprasidone: Results of a 28‐week double‐blind study in patients with schizophrenia. American Journal of Psychiatry 2005;162:1879‐87. - PubMed
-
- Phillips GA, Brunt DL, Roychowdhury SM, Xu W, Naber D. The relationship between quality of life and clinical efficacy from a randomized trial comparing olanzapine and ziprasidone. Journal of Clinical Psychiatry 2006;67(9):1397‐403. - PubMed
Canive 2000 {published data only}
-
- Canive JM, Edgar JC, LaNoue MD, Miller GA, Weisend MP, Tuason VB. A magnetoencephalographic examination on the effects of olanzapine and risperidone in patients with schizophrenia. Proceedings of the 40th Annual Meeting of the New Clinical Drug Evaluation Unit; 2000 May 30 ‐ Jun 2; Boca Raton, Florida, USA. USA, 2000.
-
- Canive JM, Miller GA, Irwin JG, Moses SN, Thoma RJ, Edgar JC, Sherwood A, Torres F, LaNoue M, Lewis S, Hanlos F, Weisend MP, Mead V, Tuason VB. Efficacy of olanzapine and risperidone in schizophrenia: A randomized double‐blind crossover design. Psychopharmacology Bulletin 2000;39(1):105‐66. - PubMed
CN138003 {published data only}
-
- CN138003. A multicenter, double‐blind, randomized, comparative study of aripiprazole and olanzapine in the treatment of patients with acute schizophrenia. Clinical Study Report 2005.
Conley 2001 {published data only}
-
- Conley RR, Mahmoud R. A randomized double‐blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. American Journal of Psychiatry 2001; Vol. 158, issue 5:765‐74. - PubMed
-
- Conley RR, Mahmoud R. Risperidone and olanzapine in people with schizophrenia or schizoaffective disorder: a randomised double‐blind study (as supplied 2001). Data on file. Baltimore, USA: Maryland Psychiatric Research Center. - PubMed
-
- Conley RR, Mahmoud R, =Risperidone Study Group. Risperidone versus olanzapine in patients with schizophrenia and schizoaffective disorder. Proceedings of the 10th Biennial Winter Workshop on Schizophrenia; 2000 Feb 5‐11; Davos, Switzerland. 2000.
-
- Conley RR, Mahmoud R, Risperidone Study Group. Risperidone versus olanzapine in patients with schizophrenia and schizoaffective psychosis [Risperidon versus olanzapin bei patienten mit schizophrenie und schizoaffektiven psychosen]. Nervenheilkunde 2000;19(5):110‐2.
-
- Harvey PD, Green MF, McGurk SR, Meltzer HY. Changes in cognitive functioning with risperidone and olanzapine treatment: a large‐scale, double‐blind, randomized study. Psychopharmacology 2003;169(3‐4):404‐11. - PubMed
Conley 2003 {published data only}
-
- Kelly DL, Conley RR, Richardson CM, Tamminga CA, Carpenter Jr WT. Adverse effects and laboratory parameters of high‐dose olanzapine vs. clozapine in treatment‐resistant schizophrenia. Annals of Clinical Psychiatry 2003;15(3‐4):181‐6. - PubMed
Dollfus 2005 {published data only}
-
- Dollfus S. The treatment of post‐psychotic depression. European College of Neuropsychopharmacology 2006;16(Suppl 4):S165.
-
- Dollfus S, Olivier V, Chabot B, Deal C, Perrin E. Olanzapine versus risperidone in the treatment of post‐psychotic depression in schizophrenic patients. Schizophrenia Research 2005;78(2‐3):157‐9. - PubMed
Dolnak 2001 {published data only}
-
- Dolnak R, Rapaport MH. A prospective, randomized, doubleblind study examining functioning in schizophrenic patients treated with olanzapine and risperidone. Schizophrenia Research 2001;49(1, 2):225‐6.
Gureje 2003 {published data only}
-
- Gureje O, Miles W, Keks N, Grainger D, Lambert T, McGrath J, Tran P, Catts S, Fraser A, Hustig H, Andersen S, Crawford AM. Olanzapine vs risperidone in the management of schizophrenia: a randomized double‐blind trial in Australia and New Zealand. Schizophrenia Research 2003;61(2‐3):303‐14. - PubMed
Jeste 2003 {published data only}
-
- Harvey PD, Napolitano JA, Mao L, Gharabawi G. Comparative effects of risperidone and olanzapine on cognition in elderly patients with schizophrenia or schizoaffective disorder. International Journal of Geriatric Psychiatry 2003;18(9):820‐8. - PubMed
-
- Jeste DV, Barak Y, Madhusoodanan S, Grossman F, Gharabawi G. International multisite double‐blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. American Journal of Geriatric Psychiatry 2003;11(6):638‐47. - PubMed
-
- Tune L, Mulsant B, Gharabawi G. Anticholinergic effect of atypical antipsychotics in elderly patients. European College of Neuropsychopharmacology 2002;12(Suppl 3):S314.
Keefe 2006 {published data only}
-
- Keefe RSE, Young CA, Rock SL, Purdon SE, Gold JM, Breier A. One‐year double‐blind study of the neurocognitive efficacy of olanzapine, risperidone, and haloperidol in schizophrenia. Schizophrenia Research 2006;81(1):1‐15. - PubMed
-
- Lysaker PH. Olanzapine and risperidone may improve neurocognition more than haloperidol in people with schizophrenia who continue treatment for 52 weeks. Evidence‐Based Mental Health 2006;9(3):71. - PubMed
Kinon 2006a {published data only}
-
- Kinon BJ, Lipkovich I, Edwards SB, Adams DH, Ascher‐Svanum H, Siris SG. A 24‐week randomized study of olanzapine versus ziprasidone in the treatment of schizophrenia or schizoaffective disorder in patients with prominent depressive symptoms. Journal of Clinical Psychopharmacology 2006;26(2):157‐62. - PubMed
Kinon 2006b {published data only}
-
- Kinon BJ, Noordsy DL, Liu‐Seifert H, Gulliver AH, Ascher‐Svanum H, Kollack‐Walker S. Randomized, double‐blind 6‐month comparison of olanzapine and quetiapine in patients with schizophrenia or schizoaffective disorder with prominent negative symptoms and poor functioning. Journal of Clinical Psychopharmacology 2006;26(5):453‐61. - PubMed
Krakowski 2006 {published data only}
-
- Krakowski MI. Clozapine and olanzapine in violent schizophrenics. CRISP database (https://www‐commons.cit.nih.gov/crisp/index.html (accessed 19th February 2001).
-
- Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Archives of General Psychiatry 2006;63(6):622‐9. - PubMed
Kumra 2007 {published data only}
-
- Kumra S, Kranzler H, Gerbino‐Rosen G, Kester HM, DeThomas C, Kafantaris V, Correll CU, Kane JM. Clozapine and "high‐dose" olanzapine in refractory early‐onset schizophrenia: A 12‐week randomized and double‐blind comparison. Biological Psychiatry 2007;4:1‐6. - PubMed
Lecrubier 2006 {published data only}
-
- Lecrubier Y, Quintin P, Bouhassira M, Perrin E, Lancrenon S. The treatment of negative symptoms and deficit states of chronic schizophrenia: olanzapine compared to amisulpride and placebo in a 6‐month double‐blind controlled clinical trial. Acta Psychiatrica Scandinavica 2006;114:319‐27. - PubMed
Lieberman 2005 {published data only}
-
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353(12):1209‐23. - PubMed
McEvoy 2006 {published data only}
-
- McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. American Journal of Psychiatry 2006;163(4):600‐10. - PubMed
McEvoy 2007 {published data only}
-
- Keefe RSE, Gu H, Sweeney JA, Perkins DO, McEvoy JP, Hamer RM, Lieberman JA. The effects of olanzapine, quetiapine and risperidone on neurocognitive function in first‐episode psychosis: a double‐blind 52‐week comparison. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
-
- Lieberman J, McEvoy JP, Perkins D, Hamer RH. Comparison of atypicals in first‐episode psychosis: a randomized, 52‐week comparison of olanzapine, quetiapine, and risperidone. Journal of the European College of Neuropsychopharmacology 2005;15(Suppl 3):S525.
-
- McEvoy JP, Lieberman JA, Perkins DO, Hamer RM, Gu H, Lazarus A, Sweitzer D, Olexy C, Weiden P, Strakowski SD. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: A randomized, double‐blind 52‐week comparison. American Journal of Psychiatry 2007;164:1050‐60. - PubMed
-
- McEvoy JP, Perkins DO, Gu H, Hamer RM, Lieberman JA. Clinical effectiveness and predictors of treatment non‐adherence: comparison of olanzapine, quetiapine, and risperidone in first‐episode psychosis. Schizophrenia Research 2006;86(Suppl 1):S130. - PubMed
-
- McEvoy JP, Perkins DO, Gu H, Hamer RM, Lieberman JA. Olanzapine, quetiapine, and risperidone in the treatment of first‐episode psychosis: effectiveness and factors influencing adherence to treatment. European College of Neuropsychopharmacology 2006;16(Suppl 4):S425.
McQuade 2004 {published data only}
-
- Jody D, Mcquade Rd, Kujawa M, Carson W, Iwamoto T, Archibald D, Stock E. Long‐term weight effects of aripiprazole versus olanzapine. Schizophrenia Research 2004;67(1):187.
-
- Kujawa MJ, McQuade RD, Jody DN, Carson WH, Abou‐Gharbia N, Iwamoto T, Archibald DG, Stock EG. Long‐term weight effects of aripiprazole vs olanzapine in a 26‐week, double‐blind study. Proceedings of the XXIVth Collegium Internationale Neuro‐Psychopharmacologicum Congress; 2004 June 20–24, Paris, France 2004.
-
- McQuade RD, Stock E, Marcus R, Jody D, Gharbia NA, Vanveggel S, Carson WH. A comparison of weight change during treatment with olanzapine or aripiprazole: Results from a randomized, double‐blind study. Journal of Clinical Psychiatry 2004;65(Suppl 18):47‐56. - PubMed
Meltzer 2003 {published data only}
-
- Alphs L, Anand R, Islam MZ, Meltzer HY, Kane JM, Krishnan R, Green AI, Potkin S, Chouinard G, Lindenmayer JP, Kerwin R. The International Suicide Prevention Trial (InterSePT): Rationale and design of a trial comparing the relative ability of clozapine and olanzapine to reduce suicidal behavior in schizophrenia and schizoaffective patients. Schizophrenia Bulletin 2004;30(3):577‐86. - PubMed
-
- Bourgeois M, Swendsen J, Young F, Amador X, Pini S, Cassano GB, Lindenmayer JP, Hsu C, Alphs L, Meltzer HY, InterSePT Study Group. Awareness of disorder and suicide risk in the treatment of schizophrenia: results of the international suicide prevention trial. American Journal of Psychiatry 2004;161(8):1494‐6. - PubMed
-
- Glick ID, Zaninelli R, Hsu C, Young FK, Weiss L, Gunay I, Kumar V. Patterns of concomitant psychotropic medication use during a 2‐year study comparing clozapine and olanzapine for the prevention of suicidal behavior. Journal of Clinical Psychiatry 2004;65(5):679‐85. - PubMed
-
- Meltzer HY, Alphs L, Greem AI, Altamura AC, Anand R, Bertoldi A, Bourgeois M, Chouinard G, Islam MZ, Kane J, Krishnan R, Lindenmayer J‐P, Potkin S, InterSePT Study Group. Clozapine treatment for suicidality in schizophrenia. Archives of General Psychiatry 2003;60:82‐91. - PubMed
-
- Potkin SG, Alphs L, Hsu C, Krishn NK, Ranga R, Anand R, Young FK, Meltzer H, Green A. Predicting suicidal risk in schizophrenic and schizoaffective patients in a prospective two‐year trial. Biological Psychiatry 2003;54(4):444‐52. - PubMed
Moresco 2004 {published data only}
-
- Moresco RM, Cavallaro R, Messa C, Bravi D, Gobbo C, Galli LLG, Colombo C, Rizzo G, Velona I, Smeraldi E, Fazio F. Cerebral D2 and 5‐HT2 receptor occupancy in Schizophrenic patients treated with olanzapine or clozapine. Journal of Psychopharmacology 2004;18(3):355‐65. - PubMed
Mori 2004 {published data only}
-
- Mori K, Nagao M, Yamashita H, Morinobu S, Yamawaki S. Effect of switching to atypical antipsychotics on memory in patients with chronic schizophrenia. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2004;28(4):659‐65. - PubMed
Mortimer 2004 {published data only}
-
- Mortimer A. The European First Episode Schizophrenia Trial: comparison of outcome in first episode schizophrenia with different low dose antipsychotic regimens (EUFEST). National Research Register 2003; Vol. 1.
-
- Mortimer A, Martin S, Loo H, Peuskens J, SOLIANOL Sudy Group. A double‐blind, randomized comparative trial of amisulpride versus olanzapine for 6 months in the treatment of schizophrenia. International Clinical Psychopharmacology 2004;19(2):63‐9. - PubMed
Naber 2005 {published data only}
-
- Bender S, Dittmann‐Balcar A, Schall U, Wolstein J, Klimke A, Riedel M, Vorbach E‐U, Kuhn K‐U, Lambert M, Dittmann RW, Naber D. Influence of atypical neuroleptics on executive functioning in patients with schizophrenia: a randomized, double‐blind comparison of olanzapine vs clozapine. International Journal of Neuropsychopharmacology 2006;9(2):135‐45. - PubMed
-
- Naber D, Riedel M, Klimke A, Vorbach E‐U, Lambert M, Kühn K‐U, Bender S, Bandelow B, Lemmer W, Moritz S, Dittmann RW. Randomized double blind comparison of olanzapine vs. clozapine on subjective well‐being and clinical outcome in patients with schizophrenia. Acta Psychiatrica Scandinavica 2005;111(2):106‐15. - PubMed
Ozguven 2004 {published data only}
-
- Ozguven HD, Oner O, Baskak B, Oner P, Atbasoglu EC. The metabolic and clinical effects of olanzapine and quetiapine: preliminary findings from a randomized single‐blind trial in patients with schizophrenia. Schizophrenia Research 2004;67(1):190‐1.
Purdon 2000 {published data only}
-
- Purdon SE, Jones BD, Stip E, Labelle A, Addington D, David SR, Breier A, Tollefson GD. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for research in schizophrenia. Archives of General Psychiatry 2000;57(3):249‐58. - PubMed
-
- Purdon SE, Woodward N, Lindborg SR, Stip E. Procedural learning in schizophrenia after 6 months of double‐blind treatment with olanzapine, risperidone, and haloperidol. Psychopharmacology 2003;169(3‐4):390‐7. - PubMed
Riedel 2007 {published data only}
-
- Riedel M, Müller N, Spellmann I, Engel RR, Musil R, Valdevit R, Dehning S, Douhet A, Cerovecki A, Strassnig M, Möller H‐J. Efficacy of olanzapine versus quetiapine on cognitive dysfunctions in patients with an acute episode of schizophrenia. European Archieves of Psychiatry and Clinical Neuroscience 2007;748:360‐70. - PubMed
Robinson 2006 {published data only}
-
- Robinson DG, Woerner MG, Napolitano B, Patel RC, Sevy SM, Gunduz‐Bruce H, Soto‐Perello JM, Mendelowitz A, Khadivi A, Miller R, McCormack J, Lorell BS, Lesser ML, Schooler NR, Kane JM. Randomized comparison of olanzapine versus risperidone for the treatment of first‐episode schizophrenia: 4‐month outcome. American Journal of Psychiatry 2006;163:2096‐102. - PubMed
Sacchetti 2004 {published data only}
-
- Sacchetti E, Valsecchi P, Regini C, Galluzzo A, Cacciani P. Comparison of quetiapine, olanzapine and risperidone in a randomized study in patients with schizophrenia. Proceedings of the Thematic Conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence, Italy. 2004.
-
- Sacchetti E, Valsecchi P, Regini C, Galluzzo A, Cacciani P, Agrimi E, Mencacci C. Comparison of quetiapine, olanzapine and risperidone in patients with schizophrenia: interim results of a randomised, rater‐blinded study. European College of Neuropsychopharmacology 2003;13(4):S350.
-
- Sacchetti E, Valsecchi P, Regini C, Galluzzo A, Cacciani P, Agrimi E, Mencacci C. Comparison of quetiapine, olanzapine, and risperidone in schizophrenia. European College of Neuropsychopharmacology 2004;14(Suppl 3):S286.
Shaw 2006 {published data only}
-
- Shaw P, Sporn A, Gogtay N, Overman GP, Greenstein D, Gochman P, Tossell JW, Lenane M, Rapoport JL. Childhood‐onset schizophrenia: a double‐blind, randomized clozapine‐olanzapine comparison. Archives of General Psychiatry 2006;63(7):721‐30. - PubMed
Sikich 2004 {published data only}
-
- Sikich L. Critical decisions in the treatment of adolescent and pediatric psychosis. 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
-
- Sikich L. Critical decisions in the treatment of adolescent and pediatric psychosis. Proceeding of the 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans, Louisiana, USA. Marathon Multimedia, 2001.
-
- Sikich L, Hamer RM, Bashford RA, Sheitman BB, Lieberman JA. A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double‐blind, randomized, 8‐week trial. Neuropyschopharmacology 2004;29(1):133‐45. - PubMed
-
- Sikich L, Williamson K, Malekpour A, Bashford RA, Hooper S, Sheitman B, Lieberman JA. Interim results of a randomized controlled trial of haloperidol, risperidone, and olanzapine in psychotic youth. Proceedings of the 38th Annual Meeting of the American College of Neuropsychopharmacology; 1999 Dec 12‐16; Acapulco, Mexico. USA, 1999.
Simpson 2004 {published data only}
-
- Harvey PD, Bowie C, Loebel AD. Long‐term cognitive improvement: ziprasidone versus olanzapine. Proceedings of the 157th Annual Meeting of the American Psychiatric Association; 2004 May 1‐6; New York, New York, USA. 2004.
-
- Harvey PD, Bowie CR, Loebel A, Warrington L. Cognitive improvement and neuropsychological normalization with ziprasidone or olanzapine: Results of a 6‐month study. European College of Neuropsychopharmacology 2004;14(Suppl 3):S294.
-
- Harvey PD, Siu CO, Romano S. Randomized, controlled, double‐blind, multicenter comparison of the cognitive effects of ziprasidone versus olanzapine in acutely ill inpatients with schizophrenia or schizoaffective disorder. Psychopharmacology 2004;172(3):324‐32. - PubMed
-
- Masand PS, Loebel AD. Analysis of remission in a six‐month double‐blind continuation study of ziprasidone versus olanzapine. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
-
- Meyer J, Nasrallah H, Loebel A, Parsons B. Comparative effects of ziprasidone and olanzapine on markers of insulin resistance: results of a 6‐week randomized study in patients with acute schizophrenia. Proceedings of the Collegium Internationale Neuro‐Psychopharmacologium 25th Biennial Congress; 2006 July 9‐13, Chicago, Illinois 2006.
Sirota 2006 {published data only}
-
- Sirota P. Quetiapine versus olanzapine for the treatment of negative symptoms in patients with schizophrenia. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006. - PubMed
-
- Sirota P, Pannet I, Koren A, Tchernichovsky E. Quetiapine versus olanzapine for the treatment of negative symptoms in patients with schizophrenia. Human Psychopharmacology 2006;21(4):227‐34. - PubMed
-
- Sirota P, Tchernichowsky E, Panet I, Koren A. The effectiveness of quetiapine versus olanzapine in improving negative symptoms of patients with schizophrenia. Schizophrenia Research 2004;67(1):170.
Stroup 2006 {published data only}
-
- Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. American Journal of Psychiatry 2006;163(4):611‐22. - PubMed
Svestka 2003a {published data only}
-
- Svestka J, Synek O, Zourkova A. Olanzapine versus risperidone in first‐episode schizophrenic and schizoform disorders: a double‐blind comparison. European College of Neuropsychopharmacology 2003;13(4):S291.
Svestka 2003b {published data only}
-
- Svestka J, Synek O, Zourkova A. A double‐blind comparison of olanzapine and quetiapine in treatment of acute exacerbations of schizophrenic or schizoaffective disorders. European College of Neuropsychopharmacology 2003;13(4):S291.
Svestka 2005 {published data only}
-
- Svestka J. Comparison of olanzapine versus ziprasidone in acute schizophrenia. Psychiatrie Prague 2005;9:4.
Tollefson 2001 {published data only}
-
- Beuzen JN, Birkett M, Kiesler G, Wood A. Olanzapine vs. clozapine in resistant schizophrenic patients ‐ results of an international double‐ blind randomised clinical trial. Proceedings of the 21st Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1998 Jul 12‐16; Glasgow, UK. 1998.
-
- Tollefson GD, Birkett MA, Kiesler GM, Wood AJ, Lilly Resistant Schizophrenia Study Group. Double‐blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biological Psychiatry 2001;49(1):52‐63. - PubMed
Tran 1997 {published data only}
-
- Ahmed S, Zhang F, Walker D, Beglinger L, Earley WR, Tran PV. Olanzapine versus risperidone for treatment of negative symptoms in schizophrenia. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California, USA. 2003.
-
- Edgell ET, Andersen SW, Johnstone BM, Dulisse B, Revicki D, Breier A. Olanzapine versus risperidone: a prospective comparison of clinical and economic outcomes in schizophrenia. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):S92. - PubMed
-
- Feldman PD, Kaiser CJ, Kennedy JS, Sutton VK, Tran PV, Tollefson GD, Zhang F, Breier A. Comparison of risperidone and olanzapine in the control of negative symptoms of chronic schizophrenia and related psychotic disorders in patients aged 50 to 65 years. Journal of Clinical Psychiatry 2003;64(9):998‐1004. - PubMed
-
- Glick ID, Berg PH. Time to study discontinuation, relapse, and compliance with atypical or conventional antipsychotics in schizophrenia and related disorders. International Clinical Psychopharmacology 2002;17(2):65‐8. - PubMed
-
- Tollefson GD, Tran PV, Hamilton S, Kuntz A. Olanzapine versus risperidone in the treatment of psychosis. Preliminary report. Biological Psychiatry 1997;41:20S.
Van Nimwegen 2006 {published data only}
-
- Nimwegen L, Haan L. Early withdrawal in a double‐blind randomized clinical trial with olanzapine and risperidone performed in adolescents with first psychosis. Psychopathology 2006;39(3):158. - PubMed
-
- Nimwegen L, Haan L, Beveren N, Laan W, Brink W, Linszen D. Obsessive compulsive symptoms in a randomized double blind. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia; 2006 Feb 6‐10; Davos, Switzerland. 2006.
-
- Nimwegen L, Haan L, Beveren N, Laan W, Brink W, Linszen D. Subjective well‐being and craving for cannabis in first psychosis, a randomized double blind comparison of olanzapine versus risperidone. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia; 2006 Feb 6‐10; Davos, Switzerland. 2006.
Vanelle 2006 {published data only}
-
- Vanelle JM, Douki S. A double‐blind randomised comparative trial of amisulpride versus olanzapine for 2 months in the treatment of subjects with schizophrenia and comorbid depression. European Psychiatry 2006;21:523‐30. - PubMed
-
- Vanelle JM, Douki S. Metabolic control in patients with comorbid schizophrenia and depression treated with amisulpride or olanzapine. European College of Neuropsychopharmacology 2004;14(Suppl 3):S284.
Volavka 2002 {published data only}
-
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. American Journal of Psychiatry 2002;159(6):1018‐28. - PubMed
-
- Citrome L, Volavka J, Czobor P, Sheitman B, Lindenmayer J‐P, McEvoy J, Cooper TB, Chakos M, Lieberman JA. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatric Services 2001;52(11):1510‐4. - PubMed
-
- Czobor P, Volavka J, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Antipsychotic‐induced weight gain and therapeutic response: A differential association. Journal of Clinical Psychopharmacology 2002;22(3):244‐51. - PubMed
-
- Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. American Journal of Psychiatry 2003;160(2):290‐6. - PubMed
-
- Lindenmayer JP, Czobor P, Volavka J, Lieberman JA, Citrome L, Sheitman B, McEvoy JP, Cooper TB, Chakos M. Effects of atypical antipsychotics on the syndromal profile in treatment‐resistant schizophrenia. Journal of Clinical Psychiatry 2004;65(4):551‐6. - PubMed
Voruganti 2007 {published data only}
-
- Voruganti LP, Awad AG, Parker G, Forrest C, Usmani Y, Fernando MLD, Senthilal S. Cognition, functioning and quality of life in schizophrenia treatment: Results of a one‐year randomized controlled trial of olanzapine and quetiapine. Schizophrenia Research 2007; Vol. 96, issue 1‐3:146‐55. - PubMed
Wagner 2005 {published data only}
-
- Quednow BB, Wagner M, Westheide J, Beckmann K, Bliesener N, Maier W, Kuhn KU. Sensorimotor gating and habituation of the startle response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biological Psychiatry 2006;59(6):536‐45. - PubMed
-
- Wagner M, Quednow BB, Westheide J, Schlaepfer TE, Maier W, Kuhn K‐U. Cognitive improvement in schizophrenic patients does not require a serotonergic mechanism: randomized controlled trial of olanzapine vs amisulpride. Neuropsychopharmacology 2005;30(2):381‐90. - PubMed
Wang 2002 {published data only}
-
- Wang C, Feng Y, Wang L. A double‐blind randomized controlled study of olanzapine and clozapine on treatment of schizophrenia. Shanghai Archives of Psychiatry 2002;14(3):143‐5.
Wang 2006 {published data only}
-
- Wang X, Savage R, Borisov A, Rosenberg J, Woolwine B, Tucker M, May R, Feldman J, Nemeroff CB, Miller AH. Efficacy of risperidone versus olanzapine in patients with schizophrenia previously on chronic conventional antipsychotic therapy: a switch study. Journal of Psychiatric Research 2006;40(7):669‐76. - PubMed
Wynn 2007 {published data only}
References to studies excluded from this review
Almond 1999 {published data only}
-
- Almond S, O'Donnell O, McKendrick J. The cost‐analysis of olanzapine compared with haloperidol and risperidone in the treatment of schizophrenia in the UK. European College of Neuropsychopharmacology 1999;9:S289.
Alvarez 2006 {published data only}
-
- Alvarez E, Ciudad A, Olivares JM, Bousono M, Gomez JC. A randomized, 1‐year follow‐up study of olanzapine and risperidone in the treatment of negative symptoms in outpatients with schizophrenia. Journal of Clinical Psychopharmacology 2006;26(3):238‐49. - PubMed
Alvarez‐Jimenez 2006 {published data only}
-
- Alvarez‐Jimenez M, Gonzalez‐Blanch C, Vazquez‐Barquero JL, Perez‐Iglesias R, Martinez‐Garcia O, Perez‐Pardal T, Ramirez‐Bonilla ML, Crespo‐Facorro B. Attenuation of antipsychotic‐induced weight gain with early behavioral intervention in drug‐naive first‐episode psychosis patients: a randomized controlled trial. Journal of Clinical Psychiatry 2006;67(8):1253‐60. - PubMed
Antonova 2005 {published data only}
-
- Antonova E, Kumari V, Halari R, Zachariah E, Mehrotra R, Kumar A, Sharma T. Superior cognitive efficacy of atypical antipsychotics olanzapine, risperidone, and quetiapine, as a group, relative to low doses of conventional antipsychotics. Schizophrenia Bulletin 2005;31:474.
Apiquian 2003 {published data only}
-
- Apiquian R, Fresan A, Herrera K, Ulloa RE, Loyzaga C, LaFuente‐Sandoval C, Gutierrez D, Nicolini H. Minimum effective doses of haloperidol for the treatment of first psychotic episode: a comparative study with risperidone and olanzapine. International Journal of Neuropsychopharmacology 2003;6(4):403‐8. - PubMed
Aquila 2000 {published data only}
-
- Aquila R, Weiden PJ, Kinon BJ, Milton DR, Zygmunt A, Swindle RW, Stauffer VL. Effectiveness of olanzapine upon psychiatric and vocational rehabilitation outcomes. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18; Chicago, Illinois, USA. 2000.
Ascher‐Svanum 2006 {published data only}
Baloescu 2006 {published data only}
-
- Baloescu A, Vasile D, Gheorghe MD, Grigorescu G. Side effects of atypical antipsychotics ‐ prediction factor for compliance. European College of Neuropsychopharmacology 2006;16(Suppl 4):S403.
Basson 2001 {published data only}
-
- Basson BR, Kinon BJ, Taylor CC, Szymanski KA, Gilmore JA, Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. Journal of Clinical Psychiatry 2001;62(4):231‐8. - PubMed
Beasley 2001 {published data only}
-
- Beasley CM, Berg PH, Dananberg J, Kwong KC, Taylor CCM, Breier A. Treatment‐emergent potential impaired glucose tolerance and potential diabetes with olanzapine compared to other antipsychotic agents and placebo. Biological Psychiatry 2001;49(8):121S.
Beasley 2003a {published data only}
-
- Beasley CM, Sowell MO, Carlson C, Mukhopadhyay N, Dananberg J, Henry R, Breier A, Cavazzoni P. Prospective evaluation of insulin sensitivity by the hyperinsulinemic, euglycemic clamp in healthy volunteers treated with olanzapine, risperidone or placebo. Schizophrenia Research 2003;60(1):309. - PubMed
Beasley 2003b {published data only}
-
- Beasley CM, Sutton VK, Hamilton SH, Walker DJ, Dossenbach M, Taylor CC, Alaka KJ, Bykowski D, Tollefson GD. A double‐blind, randomized, placebo‐controlled trial of olanzapine in the prevention of psychotic relapse. Journal of Clinical Psychopharmacology 2003;23(6):582‐94. - PubMed
Bera 2001 {published data only}
-
- Bera RB. A comparison of patient satisfaction between seroquel, olanzapine and risperidal. Schizophrenia Research 2001;49(1, 2):220.
Beuzen 2005 {published data only}
-
- Beuzen J‐N, Pans M, Modell S, Hagens P, McQuade R, Iwamoto T, Carson W. Naturalistic study of aripiprazole treatment. Proceedings of the XIII World Congress of Psychiatry; 2005 10‐15th Sept; Cairo, Egypt. 2005.
Bitter 2005 {published data only}
-
- Bitter I, Basson BR, Dossenbach M. Antipsychotic treatment and sexual functioning in first‐time neuroleptic‐treated schizophrenic patients. International Clinical Psychopharmacology 2005;20:19‐21. - PubMed
Blonde 2004 {published data only}
-
- Blonde L, Ray S, Corey‐Lisle PK, Cislo PR, L'Italien G. The risk of new‐onset type 2 diabetes and coronary heart disease in chronic schizophrenic patients treated with aripiprazole and olanzapine. European College of Neuropsychopharmacology 2004;14(Suppl 3):S275.
Boylan 2004 {published data only}
-
- Boylan LS, Labovitz DL. Unbalanced statistical analysis of combined divalproex and antipsychotic therapy for schizophrenia. Neuropyschopharmacology 2004;29(3):636. - PubMed
Briken 2002 {published data only}
-
- Briken P, Nika E, Moritz S, Haasen C, Perro C, Yagdiran O, Naber D, Krausz M. Effect of zotepine, olanzapine and risperidone on hostility in schizophrenic patients. Schizophrenia Research 2002;57:311‐13. - PubMed
Cao 2005 {published data only}
-
- Cao D, Xie S‐P, Chen Q‐B, Yuan Y‐G, Fang Q. Characteristics of the sexual disturbance caused by chlorpromazine, risperidone, quetiapine and olanzapine and their associations with the changes of blood glucose and blood lipids in male patients with schizophrenia. Chinese Journal of Clinical Rehabilitation 2005;9(36):63‐8.
Casey 2003 {published data only}
-
- Casey D, L'Italien G, Waldeck R, Cislo P, Carson W. Metabolic syndrome comparison between olanzapine, aripiprazole, and placebo. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17–22; San Francisco, California, USA 2003.
Chaudhry 2006 {published data only}
-
- Chaudhry HR, Niaz S, Arshad N, Peracha F, Ayub A, Mufti KA. Comparison of risperidone, olanzapine and quetiapine in relation to body weight, serum blood glucose and prolactin levels. European College of Neuropsychopharmacology 2006;16(Suppl 4):S241.
Chen 2003 {published data only}
-
- Chen F, Liang L, Zhu XH. A control study of elderly patients with schizophrenia treated with olanzapine or clozapine. Journal of Clinical Psychological Medicine 2003;13(5):298‐9.
Chen 2005 {published data only}
-
- Chen J, Li Z. A controlled study of olanzapine versus clozapine in schizophrenia. Journal of Clinical Psychosomatic Diseases 2005;11(3):217‐8.
Chrzanowski 2006 {published data only}
-
- Chrzanowski WK, Marcus RN, Torbeyns A, Nyilas M, McQuade RD. Effectiveness of long‐term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52‐week, open‐label comparison with olanzapine. Psychopharmacology 2006;189(2):259‐66. - PubMed
Citrome 2004 {published data only}
-
- Citrome L, Casey DE, Daniel DG, Wozniak P, Kochan LD, Tracy KA. Adjunctive divalproex and hostility among patients with schizophrenia receiving olanzapine or risperidone. Psychiatric Services 2004;55(3):290‐4. - PubMed
Ciudad 2004 {published data only}
-
- Ciudad A, Álvarez E, Bousoño M, Cuesta M, Gómez JC, Olivares JM. Olanzapine versus risperidone: Results of a one‐year randomized trial in outpatients with schizophrenia with prominent negative symptoms. Proceedings of the 11th Biennial Winter Workshop on Schizophrenia; 2004 Feb 7‐14; Davos, Switzerland. 2004.
Conley 1999 {published data only}
-
- Conley J, Goldman RS, Bilder RM, Bates J, Reiter G, Pappadopulos E, Robinson D, Alvir JMA, Liebrman J, Schooler N. A comparison of the neurocognitive effects of treatment with typical and atypical neuroleptics in first‐episode schizophrenia. Schizophrenia Research 1999;36(1‐3):128.
Cornblatt 2002 {published data only}
-
- Cornblatt B, Kern RS, Carson WH, Ali MW, Luo X, Green M. Neurocognitive effects of aripiprazole versus olanzapine in stable psychosis. International Journal of Neuropsychopharmacology 2002;5(Suppl 1):S185.
Crespo‐Facorro 2006 {published data only}
-
- Crespo‐Facorro B, Perez‐Iglesias R, Ramirez‐Bonilla M, Martinez‐Garcia O, Llorca J, Vazquez‐Barquero JL. A practical clinical trial comparing haloperidol, risperidone, and olanzapine for the acute treatment of first‐episode nonaffective psychosis. Journal of Clinical Psychiatry 2006;67(10):1511‐21. - PubMed
Czekalla 2001 {published data only}
-
- Czekalla J, Beasley CM Jr, Dellva MA, Berg PH, Grundy S. Analysis of the qtc interval during olanzapine treatment of patients with schizophrenia and related psychosis. Journal of Clinical Psychiatry 2001;62(3):191‐8. - PubMed
Dai 2004a {published data only}
-
- Dai J‐P, Zhao Z‐H, Mai G‐Y. Comparative study on the effect of olanzapine and seroquel of schizophrenia. Chinese Journal of Behavioral Medical Science 2004;13(3):291‐93.
Dai 2004b {published data only}
-
- Dai J‐P, Zhao Z‐H, Mai G‐Y. Comparative study on the influence of olanzapine and clozapine on efficacy and quality of life in schizophrenia. Chinese Journal of Behavioral Medical Science 2004;13(4):396‐98.
Dakhale 2005 {published data only}
-
- Dakhale GN, Khanzode SD, Khanzode SS, Saoji A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology 2005;182(4):494‐8. - PubMed
David 2000a {published data only}
-
- David SR, Taylor CC, Kinon BJ, Breier A. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clinical Therapeutics 2000;22(9):1085‐96. - PubMed
David 2000b {published data only}
-
- David SR, Meehan KM, Sutton VK, Taylor CC. Treatment of negative symptoms with olanzapine in comparison with other novel antipsychotic agents. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):s140.
De Haan 2002 {published data only}
-
- Haan L, Beuk N, Hoogenboom B, Dingemans P, Linszen D. Obsessive‐compulsive symptoms during treatment with olanzapine and risperidone: a prospective study of 113 patients with recent‐onset schizophrenia or related disorders. Journal of Clinical Psychiatry 2002;63(2):104‐7. - PubMed
Deng 2000 {published data only}
-
- Deng H, Zheng H, He Z. A comparative trial of olanzapine versus clozapine in the treatment of schizophrenia. Shanghai Archives of Psychiatry 2000;12(3):143‐45.
Dossenbach 2005 {published data only}
-
- Dossenbach M, Arango‐Dávila C, Ibarra HS, Landa E, Aguilar J, Caro O, Leadbetter J, Assuncao S. Response and relapse in patients with schizophrenia treated with olanzapine, risperidone, quetiapine, or haloperidol: 12‐month follow‐up of the Intercontinental Schizophrenia Outpatient Health Outcomes (IC‐SOHO) study. Journal of Clinical Psychiatry 2005;66:1021‐30. - PubMed
Ertugrul 2006 {published data only}
-
- Ertugrul A, Anil Yagcioglu AE, Woodward ND, Jayathilake K, Meltzer HY. Genetic predictors of cognitive function and response to treatment in schizophrenia. European College of Neuropsychopharmacology 2006;16(Suppl 4):S406.
Fleischhacker 2005 {published data only}
-
- Fleischhacker WW, Keet IPM, Kahn RS. The European First Episode Schizophrenia Trial (EUFEST): rationale and design of the trial. Schizophrenia Research 2005;78(2‐3):147‐56. - PubMed
García 2006 {published data only}
-
- García MC, Vidal M, Ramos R. Sexual side effects of antipsychoticcs and treatment adherence. European College of Neuropsychopharmacology 2006;16(Suppl 4):S378.
Goldberg 2000 {published data only}
-
- Goldberg TE, Dodge M, Aloia M, Egan MF, Weinberger DR. Effects of neuroleptic medications on speech disorganization in schizophrenia: biasing associative networks towards meaning. Psychological Medicine 2000;30(5):1123‐30. - PubMed
Harrigan 2004 {published data only}
-
- Harrigan EP, Miceli JJ, Anziano R, Watsky E, Reeves KR, Cutler NR, Sramek J, Shiovitz T, Middle M. A randomized evaluation of the effects of six antipsychotic agents on QTC, in the absence and presence of metabolic inhibition. Journal of Clinical Psychopharmacology 2004;24(1):62‐9. - PubMed
Harrison 2004 {published data only}
-
- Harrison D, Leaderer M, Loebel A, Murray S. Ziprasidone vs. olanzapine: change in coronary heart disease risk during a 6‐week trial. Proceedings of the thematic conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence, Italy. 2004.
Heresco‐Levy 2005 {published data only}
-
- Heresco‐Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar GCS, Ermilov M. D‐serine efficacy as add‐on pharmacotherapy to risperidone and olanzapine for treatment‐refractory schizophrenia. Biological Psychiatry 2005;57(6):577‐85. - PubMed
Hrdlicka 2001 {published data only}
-
- Hrdlicka M, Rosillon D, Duchesne I. Czech results of the RODOS study: comparison of risperidone and olanzapine from the point of view of efficacy, tolerability and treatment costs [Ceske vysledky studie RODOS: Porovnani risperidonu a olanzapinu z hlediska ucinnosti, snasenlivosti a nakladu lecby]. Ceska A Slovenská Psychiatrie 2001;97(7):343‐9.
Huber 2004 {published data only}
-
- Huber TJ, Borsutzky M, Schneider U, Emrich HM. Psychotic disorders and gonadal function: Evidence supporting the oestrogen hypothesis. Acta Psychiatrica Scandinavica 2004;109(4):269‐74. - PubMed
Karow 2002 {published data only}
-
- Karow A, Naber D. Subjective well‐being and quality of life under atypical antipsychotic treatment. Psychopharmacology 2002;162:3‐10. - PubMed
Keks 2006 {published data only}
-
- Keks NA, Tonso M, Tabone K, Mchugh M, Thomas R, Tune P, Gelman M. Clinical experience with atypical antipsychotics in an acute inpatient unit: Focus on quetiapine. International Journal of Psychiatry in Clinical Practice 2006;10(2):1‐4. - PubMed
Kelemen 2006 {published data only}
-
- Kelemen O, Nagy O, Máttyássy A, Kiss I, Janka Z, Kéri S. Do second‐generation antipsychotics disrupt decision‐making abilities in schizophrenia?. European College of Neuropsychopharmacology 2006;16(Suppl 4):S430.
Kern 2006 {published data only}
-
- Kern RS, Green MF, Cornblatt B, Owen JR, McQuade RD, Carson WH, Ali M, Marcus R. The neurocognitive effects of aripriprazole: an open‐label comparison with olanzapine. Psychopharmacology 2006;187:312‐20. - PubMed
Kim 2004 {published data only}
-
- Kim JG, Cho DH, Choi HK, Kim HJ, Cho JH, Kang SH, Lee SJ, Lee JG, Kim HT. The comparison of risperidone, olanzapine and quetiapine in the treatment of chronic schizophrenia and schizoaffective disorder. European College of Neuropsychopharmacology 2004;14(Suppl 3):S245.
Kinon 2001 {published data only}
-
- Kinon BJ, Roychowdhury SM, Milton DR, Hill AL. Effective resolution with olanzapine of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia. Journal of Clinical Psychiatry 2001;62(Suppl 2):17‐21. - PubMed
Kolff 2000 {published data only}
-
- Kolff M, Coenen A, Dis H, Duigemans P. Differential effects of antipsychotic drugs on clinical symptoms and cognitive functions in the treatment of schizophrenia. European College of Neuropsychopharmacology 2000;10(Suppl 2):S59.
Kores 2003 {published data only}
-
- Kores Plesnicar B, Zalar B, Tomori M, Krajnc I. Measurement of simple reaction time in antipsychotic treatment of patients with schizophrenia. Wiener Klinische Wochenschrift 2003;115(1‐2):58‐62. - PubMed
Kropp 2004 {published data only}
-
- Kropp S, Grohmann R, Hauser U, Rüther E, Degner D. Hyperglycemia associated with antipsychotic treatment in a multicenter drug safety project. Pharmacopsychiatry 2004; Vol. 37, issue 79‐83. - PubMed
Lee 2006 {published data only}
-
- Lee C, Wu K‐H, Habil H, Dyachkova Y, Lee P. Treatment with olanzapine, risperidone or typical antipsychotic drugs in Asian patients with schizophrenia. Australian and New Zealand Journal of Psychiatry 2006;40(5):437‐45. - PubMed
Lin 2005 {published data only}
-
- Lin Z‐Y, Li F‐Q, Yu Y‐Y, Zhong T‐P. A controlled study of olanzapine and risperidol in the treatment of elderly patients with schizophrenia. Hainan Medical Journal 2005;16(8):37‐8.
Lipkovich 2005 {published data only}
-
- Lipkovich I, Baron D, Houston J, Ahl J, Rotelli M. Flexible‐dose clinical trials: predictors and outcomes of antipsychotic dose adjustments. Journal of Clinical Psychopharmacology 2005;25(4):381‐6. - PubMed
Littrell 1999 {published data only}
-
- Littrell KH. Patients switched from depot antipsychotics to oral risperidone or olanzapine: an open‐label randomized trial. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20; Washington DC, USA. 1999.
Liu 2004 {published data only}
-
- Liu W, Li H, Zheng L. A controlled study on olanzapine and clozapine in the treatment of the acute phase of schizophrenia. Shanghai Archives of Psychiatry 2004;16(5):282‐84.
Loza 2005 {published data only}
-
- Loza B, Bartyzel M, Matysiewicz W, Mazurek I, Mosiolek A, Opielak G, Varghese S. Hyperprolactinemia during schizophrenia treatment with atypical antipsychotics: oral risperidone, depot risperidone, and oral olanzapine. European College of Neuropsychopharmacology 2005;15(Suppl 3):S522.
Malla 2004 {published data only}
-
- Malla A, Norman R, Scholten D, Townsend L, Manchanda R, Takhar J, Haricharan R. A comparison of two novel antipsychotics in first episode non‐affective psychosis: one‐year outcome on symptoms, motor side effects and cognition. Psychiatry Research 2004;129:159‐69. - PubMed
Malyarov 1999 {published data only}
-
- Malyarov S, Dzub G. Comparative assessment of the positive and negative symptom dynamics in schizophrenic patients treated with atypical antipsychotics or haloperidol. European College of Neuropsychopharmacology 1999;9:S296.
Mazurek 2003 {published data only}
-
- Mazurek I, Loza B, Lecyk A. Atypical versus typical antipsychotic treatment prognosis based on veps and wcst scores in paranoid schizophrenia. European College of Neuropsychopharmacology 2003;13(4):S347.
Meltzer 2002 {published data only}
-
- Meltzer HY, Gilliam JH, Nasdahl C. Olanzapine causes greater increases in serum lipids than risperidone. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
Moritz 2002 {published data only}
-
- Moritz S, Woodward TS, PERSIST Study Group, Krausz M, Naber D. Relationship between neuroleptic dosage and subjective cognitive dysfunction in schizophrenic patients treated with either conventional or atypical neuroleptic medication. International Clinical Psychopharmacology 2002;17:41‐44. - PubMed
Mortimer 2002 {published data only}
-
- Mortimer A. Randomised prospective parallel group comparison of clozapine vs olanzapine in the reduction of suicidality in patients with schizophrenia/schizoaffective disorder. National Research Register 2002; Vol. 1.
Musil 2006 {published data only}
-
- Musil RL, Spellmann I, Riedel M, Douhet A, Dehning S, Maino K, Zill P, Müller N, Möller HJ, Bondy B. SNAP‐25 gene polymorphisms and weight gain in schizophrenic patients treated with atypical antipsychotics. European College of Neuropsychopharmacology 2006;16(Suppl 4):S415.
Naber 2001 {published data only}
-
- Naber D, Moritz S, Lambert M, Pajonk F, Holzbach R, Mass R, Andresen B. Improvement of schizophrenic patients' subjective well‐being under atypical antipsychotic drugs. Schizophrenia Research 2001;50:79‐88. - PubMed
Naber 2002 {published data only}
-
- Naber D, Karow A, Lambert M. Psychosocial outcomes in patients with schizophrenia: quality of life and reintegration. Current Opinion in Psychiatry 2002;15:31‐6.
Newcomer 2006 {published data only}
-
- Newcomer JW, L'Italien G, Vester‐Blockland, McQuade RD, Carson WH, Marcus RN. Improvement of non‐hdl cholesterol levels among patients randomized to aripiprazole versus olanzapine. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
Oliemeulen 2000 {published data only}
-
- Oliemeulen EAP, Hoof JJM, Jogem‐Kosterman BJM, Hulsttijn W, Tuynman‐Qua HG. Is olanzapine a substitute for clozapine? The effects on psychomotor performance. Schizophrenia Research 2000;41(1):187.
Opjordsmoen 2000 {published data only}
-
- Opjordsmoen S, Brunsvik S, Melle I, Dahl A, Friis S, Haahr U, Hustoft K, Johannessen JO, Larsen TK, McGlashan TH, Simonsen E, Vaglum P. A comparison between novel and traditional antipsychotics as first‐line medication in early psychosis. Proceedings of the 2nd International Conference on Early Psychosis; 2000 Mar 31 ‐ Apr 2; New York, New York, USA. 2000.
Ortega‐Soto 1997 {published data only}
-
- Ortega‐Soto H, Apiquian R, Ulloa RE, Salas M, Loyzaga C, Mendizabal A, Brunner E. Olanzapine vs risperidone. A double blind trial in Mexican patients. Proceedings of the Collegium Internationale Neuro‐Psychopharmacologicum Regional Meeting; 1997 Aug 21‐23; Acapulco, Mexico. 1997.
Pan 2006 {published data only}
-
- Pan SM, Zhao LJ. A study of olanzapine and clozapine in treatment of schizophrenia. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine 2006;16(1):32‐3.
Perro 1999 {published data only}
-
- Perro C, Naber D, Lambert M, Moritz S, Krause M. A prospective clinical comparative‐study of four atypical antipsychotic agents in the treatment of schizophrenia. Proceedings of the 11th World Congress of Psychiatry; 1999 Aug 6‐11; Hamburg, Germany. 1999; Vol. 2:13.
Peuskens 2004 {published data only}
-
- Peuskens J, Deberdt W, Brunt D, Hill A, Liu‐Seifert H, Csernansky J, Buckley P. Medication‐specific correlates of relapse risk in schizophrenia. European College of Neuropsychopharmacology 2004;14(Suppl 3):S238.
Rabinowitz 2005 {published data only}
-
- Rabinowitz J. Pattern mixture approach to comparing outcomes ‐ of benefit in guideline development. Proceedings of the 18th European College of Neuropsychopharmacology Congress; 2005 October 22‐26, Amsterdam, Netherlands. 2005.
Ray 2004 {published data only}
-
- Ray S, Corey‐Lisle PK, Cislo PR, L'Italien G, Weiden P. An economic evaluation of aripiprazole compared to olanzapine based on metabolic side‐effect profile. European College of Neuropsychopharmacology 2004;14(Suppl 3):S279.
Reznik 2004 {published data only}
-
- Reznik I, Slavkin L, Shabash E, Shaked G, Kertzman S, Spivak B, Weizman A, Kotler M. Quetiapine ('Seroquel') and olanzapine for acute treatment of patients with schizophrenia: an open‐label, comparative study. Proceedings of the XXIVth Collegium Internationale Neuro‐Psychopharmacologicum Congress; 2004 June 20–24, Paris, France. France, 2004:1‐4.
Roerig 2004 {published data only}
-
- Roerig JL, Mitchell JE, Zwaan M, Crosby RD, Gosnell BA, Pederson K. A comparison of the effects of olanzapine and risperidone versus placebo on eating behaviors and ghrelin plasma levels in normal human subjects. Proceedings of the thematic conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence, Italy. 2004.
Ryu 2006 {published data only}
-
- Ryu SH, Jang WS, Cho EY, Kim SK, Lee DS, Hong KS. Association of leptin gene polymorphism with antipsychotic drug‐induced weight gain. European College of Neuropsychopharmacology 2006;16(Suppl 4):S419.
Sanchez 2006 {published data only}
-
- Sanchez R, Kostic D, Stock E, Torbeyns AF, Kerselaers W, Nyilas M, McQuade R, Carson WH, Marcus RN. Aripiprazole vs olanzapine in patients with acutely relapsing or chronic, stable schizophrenia: a 52‐week open‐label extension study. Proceedings of the 13th Biennial Winter Workshop on Schizophrenia Research; 2006 February 4‐10, Davos, Switzerland. 2006.
Sharma 2003 {published data only}
-
- Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology 2003;169:398‐403. - PubMed
Sowell 2002 {published data only}
-
- Sowell M, Cavazzoni P, Roychowdhury SM, Breier A. Antipsychotics and diabetes: lack of evidence for a causal relationship. International Journal of Neuropsychopharmacology 2002;5(Suppl 1):S170.
Su 2005 {published data only}
-
- Su K‐P, Wu P‐L, Pariante CM. A crossover study on lipid and weight changes associated with olanzapine and risperidone. Psychopharmacology 2005;183(3):383‐6. - PubMed
Swanson 2006 {published data only}
-
- Swanson JW, Swartz MS, Dorn RA. Effectiveness of atypical antipsychotics for substance abuse in schizophrenia patients. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
Tudor 2006 {published data only}
-
- Tudor C, Ungureanu D, Gheorghe MD. Olanzapine versus ziprasidone in parenteral administration in psychotic relapse of schizophrenia. European College of Neuropsychopharmacology 2006;16(Suppl 4):S397.
Tunis 2006 {published data only}
-
- Tunis SL, Faries DE, Nyhuis AW, Kinon BJ, Ascher‐Svanum H, Aquila R. Cost‐effectiveness of olanzapine as first‐line treatment for schizophrenia: results from a randomized, open‐label, 1‐year trial. Value in Health 2006;9(2):77‐89. - PubMed
Van Bruggen 2003 {published data only}
-
- Bruggen J, Tijssen J, Dingemans P, Gersons B, Linszen D. Symptom response and side‐effects of olanzapine and risperidone in young adults with recent onset schizophrenia. International Clinical Psychopharmacology 2003;18(6):341‐6. - PubMed
Vaughan 2000 {published data only}
-
- Vaughan K, McConaghy N, Wolf C, Myhr C, Black T. Community treatment orders: Relationship to clinical care, medication compliance, behavioural disturbance and readmission. Australian and New Zealand Journal of Psychiatry 2000;34(5):801‐8. - PubMed
Wang 2003 {published data only}
-
- Wang K, Zhang K. A study of olanzapine and clozapine in the treatment of schizophrenia.. Shandong Archires of Psychiatry 2003;16(03):141‐3.
Wang 2004a {published data only}
-
- Wang X, Wen Q, Jiang F. Comparison of efficacy safety of olanzapine and risperidone in the treatment of first episode schizophrenia. International Medicine and Health Guidance News 2004;10(8):9‐11.
Wang 2004b {published data only}
-
- Wang H. Controlled study of the effect of olanzapine and clozapine on electroencephalogram of schizophrenic patients. Journal of North China Coal Medical College 2004;6(3):289‐90.
Wang 2005 {published data only}
-
- Wang Y‐B, Xu H‐C, Sun Y, Yang M‐S, Wang X‐H, Li Y‐C. Effectiveness of olanzapine in treatment of schizophrenia. Journal of Clinical Psychological Medicine 2005;15(4):224‐5.
Weickert 2003 {published data only}
-
- Weickert TW, Goldberg TE, Marenco S, Bigelow LB, Egan MF, Weinberger DR. Comparison of cognitive performances during a placebo period and an atypical antipsychotic treatment period in schizophrenia: critical examination of confounds. Neuropyschopharmacology 2003;28(8):1491‐500. - PubMed
Wolf 2002 {published data only}
-
- Wolf K, Wolf K, Mass R, Kiefer F, Wiedemann K, Naber D. Improvement of facial expression of emotions (fee) of schizophrenic patients under olanzapine versus risperidone. A prospective facial‐emg study. Proceedings of the 12th World Congress of Psychiatry; 2002 Aug 24‐29; Yokohama, Japan. 2002.
Wolf 2005 {published data only}
-
- Wolf K, Mass R, Kiefer F, Eckert K, Stritzky AV, Haasen C, Wiedemann K, Naber D. The influence of olanzapine versus risperidone on facial expression of emotions in schizophrenia‐preliminary results of a facial electromyogram study. Journal of Clinical Psychopharmacology 2005;25(3):278‐81. - PubMed
Wu 2006 {published data only}
-
- Wu R‐R, Zhao J‐P, Liu Z‐N, Zhai J‐G, Guo X‐F, Guo W‐B, Tang J‐S. Effects of typical and atypical antipsychotics on glucose‐insulin homeostasis and lipid metabolism in first‐episode schizophrenia. Psychopharmacology 2006;186(4):572‐8. - PubMed
Wyszogrodzka‐Kuchars 2006 {published data only}
-
- Wyszogrodzka‐Kucharska A, Rabe‐Jablonska J. Vitamin D deficiency and impaired parathormone metabolism as a risk factor of low bone mineral density in patients with diagnosis of schizophrenia treated with second generation antipsychotics. European College of Neuropsychopharmacology 2006;16(Suppl 4):S373.
Yagdiran 2000 {published data only}
-
- Yagdiran O, Krausz M, =PERSIST. Depressive symptoms under atypical neuroleptic treatment in schizophrenia. Nervenarzt 2000;71(Suppl 1):S135‐36.
Yamashita 2005 {published data only}
-
- Yamashita H, Mori K, Nagao M, Okamoto Y, Morinobu S, Yamawaki S. Influence of aging on the improvement of subjective sleep quality by atypical antipsychotic drugs in patients with schizophrenia: comparison of middle‐aged and older adults. American Journal of Geriatric Psychiatry 2005;13(5):377‐84. - PubMed
Yang 2003 {published data only}
-
- Yang X, Mei Q. A comparison of olanzapine and risperidone in the treatment of first‐episode schizophrenia. Shanghai Archives of Psychiatry 2003;15(6):338‐48.
Yu 2002 {published data only}
-
- Yu G, Ding G, Li X. An economic burden comparison of typical and atypical antipsychotic therapies for schizophrenia. Chinese Journal of Psychiatry 2002;35(3):177‐9.
Zelaschi 2006 {published data only}
-
- Zelaschi Sr NM, Rodriguez Sr JL, Gaitan Sr S, Palacios Vallejos Sr ME, Zieher Sr LM. The effects of the switch of conventional neuroleptics to atypical antipsychotics: a follow‐up study of patients with chronic schizophrenia. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
Zhang 2004 {published data only}
-
- Zhang HY, Lin QC, Lin JC, Guo YB. A study of olanzapine and clozapine in EEG. International Medicine and Health Guidance News 2004;10(14):113‐4.
Zheng 2001 {published data only}
-
- Zheng H, Xu CT. A clinical study of clozapine and olanzapine in treatment of resistant schizophrenia. Journal of Qiqihar Medical College 2001;22:865.
Zhong 2006 {published data only}
-
- Zhong Z‐Y, Tao J, Wang X‐L, Wu X‐L, Zhang J‐B. Effect of antipsychotic plus buflomedil hydrochlorde in ameliorating the negative symptoms of patients with schizophrenia. Zhongguo Linchuang Kangfu 2006;10(2):30‐2.
Zoccali 2003 {published data only}
-
- Zoccali R, Muscatello MR, Torre DL, Malara G, Canale A, Crucitti DDC, Spina E. Lack of a pharmacokinetic interaction between mirtazapine and the newer antipsychotics clozapine, risperidone and olanzapine in patients with chronic schizophrenia. Pharmacological Research 2003;48(4):411‐4. - PubMed
References to ongoing studies
Eli Lilly 2003a {published data only}
-
- Eli Lilly, Company. Study of olanzapine vs aripiprazole in the treatment of schizophrenia. Eli Lilly and Company Clinical Trial Registry 2003.
Eli Lilly 2003b {published data only}
-
- Eli Lilly, Company. Safety study of olanzapine and a comparator in patients with schizophrenia and schizoaffective disorder. Eli Lilly and Company Clinical Trial Registry 2003.
Eli Lilly 2004a {published data only}
-
- Eli Lilly, Company. Olanzapine versus aripiprazole in the treatment of acutely ill patients with schizophrenia. Eli Lilly and Company Clinical Trial Registry 2004.
Eli Lilly 2004b {published data only}
-
- Eli Lilly, Company. Comparison of continuing olanzapine to switching to quetiapine in overweight or obese patients with schizophrenia and schizoaffective disorder. Eli Lilly and Company Clinical Trial Registry 2004.
Eli Lilly 2006 {published data only}
-
- Eli Lilly, Company. Efficacy study of early onset of antipsychotic drug action in schizophrenia. Eli Lilly and Company Clinical Trial Registry 2006.
Mortimer 2001 {published data only}
-
- Mortimer A. A1281014 a phase IIIb, 12 week, multi‐centre, double‐blind, double‐dummy, randomised, parallel group study comparing the effects of ziprasidone versus olanzapine on quality of life in the treatment of schizophrenia and schizoaffective disorder. National Research Register 2001; Vol. 3. [N0084096621]
N0081052094 {published data only}
-
- Reveley M. Ris‐int‐45 an international, multicentre, randomised double‐blind parallel‐group trial comparing the safety and efficacy of risperidone and olanzapine in the treatment of patients with schizophrenia. National Research Register 2000. [National Research Register ]
N0081121981 {published data only}
-
- Reveley M. MREC/00/1/47 a phase IIIb multicentre randomised double‐blind trial of ziprasidone vs olanzapine in schizophrenia. National Research Register 2003; Vol. 2. [N0081121981]
NCT00001656 {published data only}
-
- Magnuson WG. Childhood onset psychotic disorders:characterization and treatment with atypical neuroleptics / Treatment of childhood onset psychotic disorders with olanzapine or clozapine. National Institutes of Health 2001.
Additional references
Altman 1996
Andreasen 1983
-
- Andeasen NC. Scale for the assessment of negative symptoms (SANS). University of Iowa, 1983.
Andreasen 1984
-
- Andreasen NC. Scale for the assessment of positive symptoms (SAPS). University of Iowa, 1984.
Anonymous 1997
-
- Anonymous. Olanzapine, sertindole and schizophrenia. Drugs and Therapeutics Bulletin 1997;35:11. - PubMed
APA 2004
-
- American Psychiatric Association. Practice guidelines for the treatment of patients with schizophrenia. 2nd Edition. American Psychiatric Association, 2004:1‐114.
Arnt 1998
-
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 1998;18:63‐101. - PubMed
Barnes 1989
-
- Barnes TR. A rating scale for drug‐induced akathisia. British Journal of Psychiatry 1989;154:672‐6. - PubMed
Bland 1997
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405‐11. - PubMed
Carpenter 1984
-
- Heinrichs DW, Hanlon ET, Carpenter WTJr. The quality of life scale: an instrument for rating the schizophrenic deficit syndrom. Schizophrenia Bulletin 1984;10:388‐96. - PubMed
Carpenter 1994
-
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. - PubMed
Chouinard 1980
-
- Chouinard G, Ross‐Chouinard A, Annable L, Jones BD. The extrapyramidal symptom rating scale. Canadian Journal of Neurological Sciences 1980;7:233.
Conley 1998
-
- Conley RR, Tamminga CA, Bartko JJ, Richardson C, Peszke M, Lingle J, Hegerty J, Love R, Gounaris C, Zaremba S. Olanzapine compared with chlorpromazine in treatment‐resistent schizophrenia. American Journal of Psychiatry 1998;155:914‐20. - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28th; Cape Town, South Africa. 2000.
Divine 1992
-
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Duggan 2005
Egger 1997
El‐Sayeh 2006
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] - PubMed
Fleischhacker 1989
-
- Fleischhacker WW, Bergmann KJ, Perovich R, Pestreich LK, Borenstein M, Lieberman JA, Kane JM. The Hillside Akathisia Skale: a new rating instrument for neuroleptic‐induced akathisia. Psychopharmacology Bulletin 1989;25:222‐6. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. - PubMed
Gaebel 2006
-
- Gaebel W, Falkai P, Weinmann S, Wobrock T. Treatment guidelines for schizophrenia [Behandlungsleitlinie Schizophrenie]. Steinkopf 2006.
Goldman 1992
-
- Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM‐IV: a review of measures of social functioning. American Journal of Psychiatry 1992;149:1148‐56. - PubMed
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy U. ECDEU assessment manual for psychopharmacology. Revised.. National Institute of MentalHealth 1976.
Heres 2006
-
- Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine. American Journal of Psychiatry 2006;163:185‐94. - PubMed
Higgins 2003
Higgins 2008
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Jayaram 2006
Jones 2006
-
- Jones PB, Barnes TRE, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on quality of life. Archives of General Psychiatry 2006;63:1079‐6. - PubMed
Kane 1988
-
- Kane JM, Honigfeld G, Singer J, Meltzer H, Clozaril Collaborative Study Group. Clozapine for the treatment of treatment‐resistant schizophrenia: a double‐blind comparison with chlorpromazine. Archives of General Psychiatry 1988;45:789‐96. - PubMed
Kane 1993
-
- Kane JM. Treatment programme and long term outcome in chronic schizophrenia. Acta Psychiatrica Scandinavica 1993;46:585‐93. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. North Tonawanda (NY): Multi‐Health Systems, 1986.
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean?. Schizophrenia Research 2005;79:231‐8. - PubMed
Leucht 2005b
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale Scores. British Journal of Psychiatry 2005;187:366‐71. - PubMed
Lewis 2006
-
- Lewis SW, Davies L, Jones P, Barnes TRE, Murray RM, Kerwin R, Taylor D, Hayhurst KP, Marwick A, Lloyd H, Dunn G. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technology Assessment 2006;10(17):iii‐iv, ix‐xi, 1‐165. - PubMed
Liebermann 2005
-
- Liebermann JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353:1209‐23. - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales ‐ a major source of bias in randomised controlled trials of treatments for schizophrenia?. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Marvaha 2004
-
- Marvaha S, Johnson S. Schizophrenia and employment ‐ a review. Social Psychiatry and Psychiatric Epidemiology 2004;39:337‐49. - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman D. The CONSORT Statement: Revised recommendations for improving the quality of reports of parrallel‐group randomized trials. JAMA 2001;285:1987‐1. - PubMed
Möller 2000
-
- Möller HJ. New assessment of atypical antipsychotics [Aktuelle Bewertung neuer/atypischer Neuroleptika]. Nervenarzt 2000;71:329‐44. - PubMed
Overall 1962
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812.
Reus 1997
-
- Reus VI. Olanzapine: a novel atypical neuroleptic agent. Lancet 1997;349:1264‐5. - PubMed
Rosenheck 1997
-
- Rosenheck R, Cramer J, Xu WC, Thomas J, Henderson W, Frisman L, Fye C, Charney D. A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. New England Journal of Medicine 1997;337:809‐15. - PubMed
Rust 1989
-
- Rust J, Golombok S. Modern Psychometrics. London: Routledge, 1989.
Simpson 1970
-
- Simpson EN, Angus JWF. A rating scale for extrapyramidal side‐effects. Acta Psychiatrica Scandinavica Supplementum 1970;212:11‐9. - PubMed
Simpson 1999
-
- Simpson GM, Josiassen RC, Stanilla JK, Leon J, Nair C, Abraham G, Odom WA, Turner RM. Double‐blind study of clozapine dose response in chronic schizophrenia. American Journal of Psychiatry 1999;156:1744‐50. - PubMed
Tandon 2008
-
- Tandon R, Keshavan MS, Nasralllah HA. Schizophrenia, "Just the Facts" What we know in 2008. 2. Epidemiology and etiology. Schizophrenia research 2008;102:1‐18. - PubMed
Tollefson 1997
-
- Tollefson GD, Beasley CM Jr, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME. Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorder: results of an international collaborative trial. American Journal of Psychiatry 1997;154(4):457‐65. - PubMed
Tsuang 1978
-
- Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality. Archives of General Psychiatry 1978;35:153‐55. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):3‐92. [MEDLINE: ] - PubMed
Van Os 2006
-
- Os J, Burns T, Cavallaro R, Leucht S, Peuskens J, Helldin L, Bernardo M, Arango C, Fleischhacker W, Lachaux B, Kane JM. Standardized remission criteria in schizophrenia. Acta Psychiatrica Scandinavica 2006;113:91‐5. - PubMed
Wahlbeck 2001
-
- Wahlbeck K, Tuunainen A, Ahokas A, Leucht S. Drop‐out rates in randomised antipsychotic drug trials. Psychopharmacology 2001;155:230‐33. - PubMed
WHO 2001
-
- WHO. The World Health report 2001‐Mental Health: New understanding, new hope. Geneva: World Health Organisation, 2001.
Xia 2007
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. The Leeds Outcomes Stakeholders Survey (LOSS) Study. Proceedings of the 15th Cochrane Colloquium; 2007 Oct 23‐27; Sao Paulo. 2007.
References to other published versions of this review
Leucht 2008
-
- Leucht S, Komossa K, Rummel‐Kluge C, Corves C, Hunger H, Schmid F, Asenjo‐Lobos C, Schwarz S, Davis JM. A meta‐analysis of head‐to‐head comparisons of second‐generation antipsychotics in the treatment of schizophrenia. American Journal of Psychiatry 2009;166:152‐63. [DOI: 10.1176/appi.ajp.2008.08030368] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

