Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women
- PMID: 20238370
- PMCID: PMC12320970
- DOI: 10.1002/14651858.CD008440
Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women
Abstract
Background: This systematic review focuses on antiretroviral therapy (ART) for treating human immunodeficiency virus (HIV) infection in ART-eligible pregnant women. Mother-to-child transmission (MTCT) is the primary means by which children worldwide acquire HIV infection. MTCT occurs during three major timepoints during pregnancy and the postpartum period: in utero, intrapartum, and during breastfeeding. Strategies to reduce MTCT focus on these periods of exposure and include maternal and infant use of ART, caesarean section before onset of labour or rupture of membranes, and complete avoidance of breastfeeding. Where these combined interventions are available, the risk of MTCT is as low as 1-2%. Thus, ART used among mothers who require treatment of HIV for their own health also plays a significant role in decreasing MTCT.This review is one in a series of systematic reviews performed in preparation for the revision of the 2006 World Health Organization (WHO) Guidelines regarding "Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants" and "Antiretroviral therapy (ART) for HIV Infections in Adults and Adolescents." The findings from these reviews were discussed with experts, key stakeholders, and country representatives at the 2009 WHO guideline review meeting. The resulting WHO 2009 "rapid advice" preliminary guidance on adult and adolescent ART now recommends lifelong treatment for all adults with HIV infection and CD4 counts <350 cells/mm(3). These recommendations also apply to pregnant women who are HIV-infected and they place a high value on early ART to benefit the mother's own health (WHO 2009). The "rapid advice" preliminary guidance also aims to minimize side effects for mothers and their infants (WHO 2009).
Objectives: Our objective was to assess the current literature regarding the treatment of HIV infection in pregnant women who are clinically or immunologically eligible for ART. This review includes an evaluation of the optimal time to start therapy in relation to the woman's laboratory parameters and/or gestational age. It also includes an analysis of which specific antiretroviral medications to start in women who are not yet on ART and which agents to continue in women who are already on ART.
Search strategy: In June 2009, electronic searches were undertaken in these databases: Cochrane's "CENTRAL," EMBASE, PubMed, LILACS, and Web of Science/Web of Social Science. Hand searches were performed of the reference lists of all pertinent reviews and studies identified. Abstracts from relevant conferences were searched. Experts in the field were contacted to locate additional studies. The search strategy was iterative.
Selection criteria: We selected randomized controlled trials and observational studies that evaluated pregnant women with HIV infection who were eligible for ART according to criteria defined by the WHO guideline review committee. Studies were included in the systematic review when a comparison group was clearly defined and where the intervention comprised triple ART. For a study to be considered, each medication in the ART regimen needed to be clearly described.
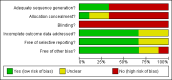
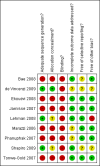
Data collection and analysis: Two authors independently assessed the selected studies for relevance and inclusion. Relevant data was then extracted from included studies, and the risk of bias assessed. In each included study, the relative risk (RR) for the intervention versus the comparison group was calculated for each outcome, as appropriate, with 95% confidence intervals (CIs).
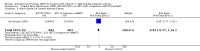
Main results: To our knowledge, there are no randomized controlled trials or observational studies that address the optimal time to start antiretroviral drugs in ART-eligible pregnant women in relation to the woman's laboratory parameters and/or gestational age. The medications to continue in ART-eligible pregnant women who are already receiving ART also have not been evaluated systematically in the current literature. The long-term mortality of HIV-positive pregnant women on ART for their own health, and the long-term virologic or clinical efficacy of ART in treating them, has not been evaluated in randomized clinical trials. In this review, surrogate outcomes for long-term mortality and virologic and clinical efficacy (e.g. MTCT and infant HIV transmission or death) were evaluated to determine the efficacy of specific antiretroviral regimens to start in women who are not yet on ART.Three randomized controlled trials and six observational studies were selected. No studies addressed comparative maternal mortality, which regimens to continue in women already on ART, or the laboratory parameters and gestational age at which to start therapy. The use of zidovudine (AZT), lamivudine (3TC) and lopinavir/ritonavir (LPV-r) starting at 28-36 weeks gestation in a breastfeeding population reduced infant HIV-transmission or death at 12 months compared to a short-course regimen (RR 0.64, 95% CI: 0.44-0.92) (deVincenzi, 2009). Starting AZT, 3TC, and nevirapine (NVP) at 34 weeks in a mixed-feeding population reduced infant HIV-transmission or death at 7 months compared to a short-course regimen (RR 0.39, 95% CI: 0.12-0.85) (Bae, 2008).In the Mma Bana study (a randomized controlled trial in a breastfeeding population) there was no difference in MTCT at six months between the AZT/3TC/LPV-r and AZT, 3TC, and abacavir (ABC) arms (RR 0.17, 95% CI: 0.02-1.44) (Shapiro, 2009). Both regimens also showed 92-95% efficacy in virologic suppression at delivery and during the breastfeeding period. In the Kesho Bora study there was a significant difference in MTCT at 12 months between breastfeeding women who initiated AZT/3TC/LPV-r starting between 28 and 36 weeks and those receiving a short course regimen (RR 0.58, 95% CI: 0.34-0.97) (deVincenzi, 2009). MTCT also decreased significantly when AZT/3TC/NVP was compared with a short-course regimen at seven months in a feeding intervention study (RR 0.15, 95% CI: 0.04-0.62) (Bae, 2008) and 12 months in a population where either exclusive breastfeeding or replacement feeding was encouraged (RR 0.14, CI: 0.04-0.47) (Ekouevi, 2008).In the Mma Bana study, there was increased risk of prematurity among infants born to women receiving AZT/3TC/LPV-r (RR 1.52, CI: 1.07- 2.17) compared with AZT/3TC/ABC (Shapiro, 2009). Ekouevi 2008 showed higher rates of infant low birth weight on AZT/3TC/NVP started at 24 weeks compared to a short course regimen started between 32 and 36 weeks (RR 1.81, 95% CI: 1.09- 3.0). Tonwe-Gold 2007 showed an increase in maternal severe adverse events among the women receiving AZT/3TC/NVP compared with a short-course regimen (RR 25.33, CI 1.49- 340.51).
Authors' conclusions: In ART-eligible pregnant women with HIV infection, ART is a safe and effective means of providing maternal virologic suppression, decreasing infant mortality, and reducing MTCT. Specifically, AZT/3TC/NVP, AZT/3TC/LPV-r, and AZT/3TC/ABC have been shown to decrease MTCT. More research is needed regarding the use of specific regimens and their maternal and infant side-effect profiles.
Conflict of interest statement
There are no conflicts of interest to report.
Figures













































References
References to studies included in this review
Bae 2008 {published data only}
de Vincenzi 2009 {published data only (unpublished sought but not used)}
-
- deVincenzi I, et al. Triple antiretroviral prophylaxis during pregnancy to prevent mother‐to‐child transmission of HIV‐1 (The Kesho Bora Study). International AIDS Society Conference. Cape Town, South Africa;July 2009:Abstract LBPEC01.
Ekouevi 2008 {published data only}
-
- Ekouevi DK, Coffie PA, Becquet R, Tonwe‐Gold B, Horo A, Thiebaut R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d'Ivoire. AIDS (London, England) 2008;22(14):1815‐20. [PUBMED: 18753864] - PubMed
Jamisse 2007 {published data only}
-
- Jamisse L, Balkus J, Hitti J, Gloyd S, Manuel R, Osman N, et al. Antiretroviral‐associated toxicity among HIV‐1‐seropositive pregnant women in Mozambique receiving nevirapine‐based regimens. Journal of acquired immune deficiency syndromes (1999) 2007;44(4):371‐6. [PUBMED: 17259905] - PubMed
Lehman 2008 {published data only}
Marazzi 2006 {published data only}
-
- Marazzi MC, Germano P, Liotta G, Guidotti G, Loureiro S, Cruz Gomes A, et al. Safety of nevirapine‐containing antiretroviral triple therapy regimens to prevent vertical transmission in an African cohort of HIV‐1‐infected pregnant women. HIV medicine 2006;7(5):338‐44. [PUBMED: 16945080] - PubMed
Phanuphak 2007 {published data only}
-
- Phanuphak N, Apornpong T, Teeratakulpisarn S, Chaithongwongwatthana S, Taweepolcharoen C, Mangclaviraj S, et al. Nevirapine‐associated toxicity in HIV‐infected Thai men and women, including pregnant women. HIV medicine 2007;8(6):357‐66. [PUBMED: 17661843] - PubMed
Shapiro 2009 {published data only (unpublished sought but not used)}
-
- Shapiro R, et al. A randomized trial comparing highly active antiretroviral therapy regimens for virologic efficacy and the prevention of mother‐to‐child transmission among breastfeeding women in Botswana (The Mma Bana Study). International AIDS Society Conference. Cape Town, South Africa;July 2009:Abstract WELBB101.
Tonwe‐Gold 2007 {published data only}
References to studies excluded from this review
Alimenti 2003 {published data only}
-
- Alimenti A, Burdge DR, Ogilvie GS, Money DM, Forbes JC. Lactic acidemia in human immunodeficiency virus‐uninfected infants exposed to perinatal antiretroviral therapy. The Pediatric infectious disease journal 2003;22(9):782‐9. [PUBMED: 14506368] - PubMed
Arrive 2007 {published data only}
-
- Arrive E, Newell ML, Ekouevi DK, Chaix ML, Thiebaut R, Masquelier B, et al. Prevalence of resistance to nevirapine in mothers and children after single‐dose exposure to prevent vertical transmission of HIV‐1: a meta‐analysis. International journal of epidemiology 2007;36(5):1009‐21. [PUBMED: 17533166] - PubMed
Arrive 2008 {published data only}
Bardeguez 2008 {published data only}
Bauer 2006 {published data only}
-
- Bauer GR, Colgrove RC, Larussa PS, Pitt J, Welles SL. Antiretroviral resistance in viral isolates from HIV‐1‐transmitting mothers and their infants. AIDS (London, England) 2006;20(13):1707‐12. [PUBMED: 16931934] - PubMed
Bellon 2004 {published data only}
-
- Bellon Cano JM, Sanchez‐Ramon S, Ciria L, Leon JA, Gurbindo D, Fortuny C, et al. The effects on infants of potent antiretroviral therapy during pregnancy: a report from Spain. Medical science monitor : international medical journal of experimental and clinical research 2004;10(5):CR179‐84. [PUBMED: 15114266] - PubMed
Black 2008 {published data only}
-
- Black V, Hoffman RM, Sugar CA, Menon P, Venter F, Currier JS, et al. Safety and efficacy of initiating highly active antiretroviral therapy in an integrated antenatal and HIV clinic in Johannesburg, South Africa. Journal of acquired immune deficiency syndromes (1999) 2008;49(3):276‐81. [PUBMED: 18845949] - PMC - PubMed
Black 2008a {published data only}
-
- Black V, Rees H. Incidence of nevirapine‐associated hepatitis in an antenatal clinic. South African medical journal = Suid‐Afrikaanse tydskrif vir geneeskunde 2008;98(2):116‐8. [PUBMED: 18350206] - PubMed
Briand 2009 {published data only}
-
- Briand N, Mandelbrot L, Chenadec J, Tubiana R, Teglas JP, Faye A, et al. No relation between in‐utero exposure to HAART and intrauterine growth retardation. AIDS (London, England) 2009;23(10):1235‐43. [PUBMED: 19424054] - PubMed
Bucceri 2002 {published data only}
-
- Bucceri AM, Somigliana E, Matrone R, Ferraris G, Rossi G, Grossi E, et al. Combination antiretroviral therapy in 100 HIV‐1‐infected pregnant women. Human reproduction (Oxford, England) 2002;17(2):436‐41. [PUBMED: 11821291] - PubMed
Bunders 2005 {published data only}
-
- Bunders MJ, Bekker V, Scherpbier HJ, Boer K, Godfried M, Kuijpers TW. Haematological parameters of HIV‐1‐uninfected infants born to HIV‐1‐infected mothers. Acta paediatrica (Oslo, Norway : 1992) 2005;94(11):1571‐7. [PUBMED: 16303696] - PubMed
Chi 2007 {published data only}
-
- Chi BH, Chintu N, Lee A, Stringer EM, Sinkala M, Stringer JS. Expanded services for the prevention of mother‐to‐child HIV transmission: field acceptability of a pilot program in Lusaka, Zambia. Journal of acquired immune deficiency syndromes (1999) 2007; Vol. 45, issue 1:125‐7. [PUBMED: 17460478] - PubMed
Ciaranello 2008 {published data only}
Clayden 2009 {published data only}
-
- Clayden, P. Safety Of Antiretrovirals In Pregnancy. Southern African Journal Of Hiv Medicine 2009;33:15‐18.
Cooper 2002 {published data only}
-
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV‐1‐infected women and prevention of perinatal HIV‐1 transmission. Journal of acquired immune deficiency syndromes (1999) 2002;29(5):484‐94. [PUBMED: 11981365] - PubMed
Cotter 2006 {published data only}
-
- Cotter AM, Garcia AG, Duthely ML, Luke B, O'Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth?. The Journal of infectious diseases 2006;193(9):1195‐201. [PUBMED: 16586354] - PubMed
Dabis 2005 {published data only}
Deschamps 2009 {published data only}
-
- Deschamps MM, Noel F, Bonhomme J, Devieux JG, Saint‐Jean G, Zhu Y, et al. Prevention of mother‐to‐child transmission of HIV in Haiti. Revista panamericana de salud publica = Pan American journal of public health 2009;25(1):24‐30. [PUBMED: 19341520] - PubMed
Dinsmoor 2002 {published data only}
Dorenbaum 2002 {published data only}
-
- Dorenbaum A, Cunningham CK, Gelber RD, Culnane M, Mofenson L, Britto P, et al. Two‐dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA : the journal of the American Medical Association 2002;288(2):189‐98. [PUBMED: 12095383] - PubMed
Duran 2006 {published data only}
-
- Duran AS, Ivalo SA, Hakim A, Masciottra FM, Zlatkes R, Adissi L, et al. Prevention of mother to child HIV transmission. Medicina 2006;66(1):24‐30. [PUBMED: 16555724] - PubMed
El 2006 {published data only}
-
- Beitune P, Duarte G. Antiretroviral agents during pregnancy: consequences on hematologic parameters in HIV‐exposed, uninfected newborn infant. European journal of obstetrics, gynecology, and reproductive biology 2006;128(1‐2):59‐63. [PUBMED: 16876310] - PubMed
El 2007 {published data only}
-
- Beitune P, Duarte G, Campbell O, Quintana SM, Rodrigues LC. Effects of antiretroviral agents during pregnancy on liver enzymes and amylase in HIV‐exposed, uninfected newborn infants. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases 2007;11(3):314‐7. [PUBMED: 17684631] - PubMed
European Collaborative Study (ECS) 2000 {published data only}
-
- European Collaborative Study. Combination antiretroviral therapy and duration of pregnancy. AIDS (London, England) 2000;14(18):2913‐20. [PUBMED: 11398741] - PubMed
European Collaborative Study (ECS) 2003 {published data only}
-
- European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV‐infected women. Journal of acquired immune deficiency syndromes (1999) 2003;32(4):380‐7. [PUBMED: 12640195] - PubMed
European Collaborative Study (ECS) 2005 {published data only}
-
- European Collaborative Study. Mother‐to‐child transmission of HIV infection in the era of highly active antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2005;40(3):458‐65. [PUBMED: 15668871] - PubMed
Fiore 2006 {published data only}
-
- Fiore S, Newell ML, Trabattoni D, Thorne C, Gray L, Savasi V, et al. Antiretroviral therapy‐associated modulation of Th1 and Th2 immune responses in HIV‐infected pregnant women. Journal of reproductive immunology 2006;70(1‐2):143‐50. [PUBMED: 16423410] - PubMed
Garcia‐Tejedor 2009 {published data only}
-
- Garcia‐Tejedor A, Maiques V, Perales A, Lopez‐Aldeguer J. Influence of highly active antiretroviral treatment (HAART) on risk factors for vertical HIV transmission. Acta obstetricia et gynecologica Scandinavica 2009;88(8):882‐7. [PUBMED: 19557554] - PubMed
Giuliano 2003 {published data only}
-
- Giuliano M, Palmisano L, Galluzzo CM, Amici R, Germinario E, Okong P, et al. Selection of resistance mutations in pregnant women receiving zidovudine and lamivudine to prevent HIV perinatal transmission. AIDS (London, England) 2003;17(10):1570‐2. [PUBMED: 12824800] - PubMed
Goetghebuer 2009 {published data only}
-
- Goetghebuer T, Haelterman E, Marvillet I, Barlow P, Hainaut M, Salameh A, et al. Vertical transmission of HIV in Belgium: a 1986‐2002 retrospective analysis. European journal of pediatrics 2009;168(1):79‐85. [PUBMED: 18392638] - PubMed
Grosch‐Woerner 2008 {published data only}
-
- Grosch‐Woerner I, Puch K, Maier RF, Niehues T, Notheis G, Patel D, et al. Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV‐1‐infected women. HIV medicine 2008;9(1):6‐13. [PUBMED: 18199167] - PubMed
Hitti 2004 {published data only}
-
- Hitti J, Frenkel LM, Stek AM, Nachman SA, Baker D, Gonzalez‐Garcia A, et al. Maternal toxicity with continuous nevirapine in pregnancy: results from PACTG 1022. Journal of acquired immune deficiency syndromes (1999) 2004;36(3):772‐6. [PUBMED: 15213559] - PubMed
Hitti 2007 {published data only}
-
- Hitti J, Andersen J, McComsey G, Liu T, Melvin A, Smith L, et al. Protease inhibitor‐based antiretroviral therapy and glucose tolerance in pregnancy: AIDS Clinical Trials Group A5084. American journal of obstetrics and gynecology 2007;196(4):331.e1‐7. [PUBMED: 17403409] - PubMed
Hoffman 2009 {unpublished data only}
-
- Hoffman R, Black V, Technau K, Merwe K, Currier J, Chersich M, Coovadia A. Impact of highly active antiretroviral therapy regimen and duration of therapy on risk of mother‐to‐child HIV transmission in Johannesburg, South Africa. International AIDS Society Conference. Capetown, South Africa;July 2009:Abstract WEPEB260.
Jackson 2007 {published data only}
-
- Jackson DJ, Chopra M, Doherty TM, Colvin MS, Levin JB, Willumsen JF, et al. Operational effectiveness and 36 week HIV‐free survival in the South African programme to prevent mother‐to‐child transmission of HIV‐1. AIDS (London, England) 2007;21(4):509‐16. [PUBMED: 17301570] - PubMed
Justman 2003 {published data only}
-
- Justman JE, Benning L, Danoff A, Minkoff H, Levine A, Greenblatt RM, et al. Protease inhibitor use and the incidence of diabetes mellitus in a large cohort of HIV‐infected women. Journal of acquired immune deficiency syndromes (1999) 2003;32(3):298‐302. [PUBMED: 12626890] - PubMed
Kourtis 2007 {published data only}
-
- Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV‐infected women and the risk of premature delivery: a meta‐analysis. AIDS (London, England) 2007;21(5):607‐15. [PUBMED: 17314523] - PubMed
Kowalska 2003 {published data only}
-
- Kowalska A, Niemiec T, Midaoui A, Burkacka E. Effect of antiretroviral therapy on pregnancy outcome in HIV‐1 positive women. Medycyna wieku rozwojowego 2003;7(4 Pt 1):459‐68. [PUBMED: 15010556] - PubMed
Livingston 2007 {published data only}
-
- Livingston EG, Cohn SE, Yang Y, Watts HD, Bardeguez AD, Jones TB, et al. Lipids and lactate in human immunodeficiency virus‐1 infected pregnancies with or without protease inhibitor‐based therapy. Obstetrics and gynecology 2007;110(2 Pt 1):391‐7. [PUBMED: 17666616] - PubMed
Lopez‐Cortes 2007 {published data only}
-
- Lopez‐Cortes LF, Ruiz‐Valderas R, Rivero A, Camacho A, Marquez‐Solero M, Santos J, et al. Efficacy of low‐dose boosted saquinavir once daily plus nucleoside reverse transcriptase inhibitors in pregnant HIV‐1‐infected women with a therapeutic drug monitoring strategy. Therapeutic drug monitoring 2007;29(2):171‐6. [PUBMED: 17417070] - PubMed
Lorenzi 1998 {published data only}
-
- Lorenzi P, Spicher VM, Laubereau B, Hirschel B, Kind C, Rudin C, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS (London, England) 1998;12(18):F241‐7. [PUBMED: 9875571] - PubMed
Marti 2007 {published data only}
-
- Marti C, Pena JM, Bates I, Madero R, Jose I, Pallardo LF, et al. Obstetric and perinatal complications in HIV‐infected women. Analysis of a cohort of 167 pregnancies between 1997 and 2003. Acta obstetricia et gynecologica Scandinavica 2007;86(4):409‐15. [PUBMED: 17486461] - PubMed
Martin 2006 {published data only}
-
- Martin F, Navaratne L, Khan W, Sarner L, Mercey D, Anderson J, et al. Pregnant women with HIV infection can expect healthy survival: three‐year follow‐up. Journal of acquired immune deficiency syndromes (1999) 2006;43(2):186‐92. [PUBMED: 16940856] - PubMed
Martin 2007 {published data only}
-
- Martin F, Taylor GP. Increased rates of preterm delivery are associated with the initiation of highly active antiretrovial therapy during pregnancy: a single‐center cohort study. The Journal of infectious diseases 2007;196(4):558‐61. [PUBMED: 17624841] - PubMed
McIntyre 2002 {published data only}
McIntyre 2006 {published data only}
-
- McIntyre J. Strategies to prevent mother‐to‐child transmission of HIV. Current opinion in infectious diseases 2006;19(1):33‐8. [PUBMED: 16374215] - PubMed
Mellins 2008 {published data only}
-
- Mellins CA, Chu C, Malee K, Allison S, Smith R, Harris L, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV‐infected women. AIDS care 2008;20(8):958‐68. [PUBMED: 18608073] - PubMed
Mofenson 2002 {published data only}
-
- Mofenson LM, Munderi P. Safety of antiretroviral prophylaxis of perinatal transmission for HIV‐infected pregnant women and their infants. Journal of acquired immune deficiency syndromes (1999) 2002;30(2):200‐15. [PUBMED: 12045684] - PubMed
Mussi‐Pinhata 2007 {published data only}
-
- Mussi‐Pinhata MM, Rego MA, Freimanis L, Kakehasi FM, Machado DM, Cardoso EM, et al. Maternal antiretrovirals and hepatic enzyme, hematologic abnormalities among human immunodeficiency virus type 1‐uninfected infants: the NISDI perinatal study. The Pediatric infectious disease journal 2007;26(11):1032‐7. [PUBMED: 17984811] - PubMed
Nurutdinova 2008 {published data only}
-
- Nurutdinova D, Onen NF, Hayes E, Mondy K, Overton ET. Adverse effects of tenofovir use in HIV‐infected pregnant women and their infants. The Annals of pharmacotherapy 2008;42(11):1581‐5. [PUBMED: 18957630] - PubMed
Onen 2008 {published data only}
-
- Onen NF, Nurutdinova D, Sungkanuparph S, Gase D, Mondy K, Overton ET. Effect of postpartum HIV treatment discontinuation on long‐term maternal outcome. Journal of the International Association of Physicians in AIDS Care (Chicago, Ill. : 2002) 2008;7(5):245‐51. [PUBMED: 18812593] - PubMed
Ono 2008 {published data only}
-
- Ono E, Nunes dos Santos AM, Menezes Succi RC, Machado DM, Angelis DS, Salomao R, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV‐1‐infected mothers on HAART. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica ... [et al.] 2008;41(8):700‐8. [PUBMED: 18797705] - PubMed
Pacheco 2006 {published data only}
-
- Pacheco SE, McIntosh K, Lu M, Mofenson LM, Diaz C, Foca M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV‐uninfected children: An analysis of the women and infants transmission study. The Journal of infectious diseases 2006;194(8):1089‐97. [PUBMED: 16991083] - PubMed
Palacios 2009 {published data only}
-
- Palacios R, Senise J, Vaz M, Diaz R, Castelo A. Short‐term antiretroviral therapy to prevent mother‐to‐child transmission is safe and results in a sustained increase in CD4 T‐cell counts in HIV‐1‐infected mothers. HIV medicine 2009;10(3):157‐62. [PUBMED: 19245537] - PubMed
Peltier 2009 {published data only}
Resino 2001 {published data only}
-
- Resino Garcia S, Bellon Cano JM, Navarro Caspistegui J, Gurbindo Gutierrez D, Leon Leal JA, Munoz‐Fernandez MA. T‐cell subsets variation during clinical and immunological progression in vertically HIV‐infected children [Variacion de las subpoblaciones de celulas T segun la progresion clinica e inmunologica en ninos infectados verticalmente por HIV‐1.]. Medicina 2001;61(5 Pt 1):557‐65. [PUBMED: 11721322] - PubMed
Schulte 2007 {published data only}
-
- Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler MG. Declines in low birth weight and preterm birth among infants who were born to HIV‐infected women during an era of increased use of maternal antiretroviral drugs: Pediatric Spectrum of HIV Disease, 1989‐2004. Pediatrics 2007;119(4):e900‐6. [PUBMED: 17353299] - PubMed
Stek 2009 {published data only}
-
- Stek AM. Antiretroviral medications during pregnancy for therapy or prophylaxis. Current HIV/AIDS reports 2009;6(2):68‐76. [PUBMED: 19358777] - PubMed
Szyld 2006 {published data only}
-
- Szyld EG, Warley EM, Freimanis L, Gonin R, Cahn PE, Calvet GA, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS (London, England) 2006;20(18):2345‐53. [PUBMED: 17117021] - PubMed
Taha 2009 {published data only}
-
- Taha TE, Kumwenda J, Cole SR, Hoover DR, Kafulafula G, Fowler MG, et al. Postnatal HIV‐1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. The Journal of infectious diseases 2009;200(10):1490‐7. [PUBMED: 19832114] - PubMed
Tang 2006 {published data only}
-
- Tang JH, Sheffield JS, Grimes J, McElwee B, Roberts SW, Laibl V, et al. Effect of protease inhibitor therapy on glucose intolerance in pregnancy. Obstetrics and gynecology 2006;107(5):1115‐9. [PUBMED: 16648418] - PubMed
Tempelman 2004 {published data only}
-
- Tempelman C, Timmermans S, Godfried MH, Dieleman JP, Boer K, Ende ME. Highly active antiretroviral therapy (HAART) in HIV‐positive pregnant women in the Netherlands, 1997‐2003: safe, effective and with few side effects [Krachtige antiretrovirale therapie (HAART) bij HIV‐positieve zwangeren in Nederland, 1997‐2003: veilig, effectief en met weinig bijwerkingen.]. Nederlands tijdschrift voor geneeskunde 2004;148(41):2021‐5. [PUBMED: 15553999] - PubMed
Thomas 2008 {unpublished data only}
-
- Thomas T, Masaba R, Ndivo R, Zeh C, Borkowf C, Thigpen M, Cock K, Amornkul P, Greenberg A, Fowler M, and Kisumu Breastfeeding Study Team. Prevention of Mother‐to‐Child Transmission of HIV‐1 among Breastfeeding Mothers Using HAART: The Kisumu Breastfeeding Study, Kisumu, Kenya, 2003‐2007. Conference on Retroviruses and Opportunistic Infections Boston MA;February 2008:Abstract 45aLB.
Thorne 2004 {published data only}
-
- Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV‐infected women treated with highly active antiretroviral therapy in Europe. AIDS (London, England) 2004;18(17):2337‐9. [PUBMED: 15577551] - PubMed
Timmermans 2005 {published data only}
-
- Timmermans S, Tempelman C, Godfried MH, Nellen J, Dieleman J, Sprenger H, et al. Nelfinavir and nevirapine side effects during pregnancy. AIDS (London, England) 2005;19(8):795‐9. [PUBMED: 15867493] - PubMed
Townsend 2006 {published data only}
-
- Townsend CL, Tookey PA, Cortina‐Borja M, Peckham CS. Antiretroviral therapy and congenital abnormalities in infants born to HIV‐1‐infected women in the United Kingdom and Ireland, 1990 to 2003. Journal of acquired immune deficiency syndromes (1999) 2006;42(1):91‐4. [PUBMED: 16763496] - PubMed
Townsend 2007 {published data only}
-
- Townsend CL, Cortina‐Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV‐infected women in the United Kingdom and Ireland. AIDS (London, England) 2007;21(8):1019‐26. [PUBMED: 17457096] - PubMed
Townsend 2009 {published data only}
-
- Townsend CL, Willey BA, Cortina‐Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and congenital abnormalities in infants born to HIV‐infected women in the UK and Ireland, 1990‐2007. AIDS (London, England) 2009;23(4):519‐24. [PUBMED: 19165088] - PubMed
Tuomala 2002 {published data only}
-
- Tuomala RE, Shapiro DE, Mofenson LM, Bryson Y, Culnane M, Hughes MD, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. The New England journal of medicine 2002;346(24):1863‐70. [PUBMED: 12063370] - PubMed
Tuomala 2005 {published data only}
-
- Tuomala RE, Watts DH, Li D, Vajaranant M, Pitt J, Hammill H, et al. Improved obstetric outcomes and few maternal toxicities are associated with antiretroviral therapy, including highly active antiretroviral therapy during pregnancy. Journal of acquired immune deficiency syndromes (1999) 2005;38(4):449‐73. [PUBMED: 15764963] - PubMed
van der Merwe 2006 {published data only}
-
- Merwe K, Chersich MF, Technau K, Umurungi Y, Conradie F, Coovadia A. Integration of antiretroviral treatment within antenatal care in Gauteng Province, South Africa. Journal of acquired immune deficiency syndromes (1999) 2006;43(5):577‐81. [PUBMED: 17031321] - PubMed
van Schalkwyk 2008 {published data only}
-
- Schalkwyk JE, Alimenti A, Khoo D, Maan E, Forbes JC, Burdge DR, et al. Serious toxicity associated with continuous nevirapine‐based HAART in pregnancy. BJOG : an international journal of obstetrics and gynaecology 2008;115(10):1297‐302. [PUBMED: 18715416] - PubMed
Vithayasai 2002 {published data only}
-
- Vithayasai V, Moyle GJ, Supajatura V, Wattanatchariya N, Kanshana S, Sirichthaporn P, et al. Safety and efficacy of saquinavir soft‐gelatin capsules + zidovudine + optional lamivudine in pregnancy and prevention of vertical HIV transmission. Journal of acquired immune deficiency syndromes (1999) 2002;30(4):410‐2. [PUBMED: 12138347] - PubMed
Volmink 2007 {published data only}
-
- Volmink J, Siegfried NL, Merwe L, Brocklehurst P. Antiretrovirals for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane database of systematic reviews (Online) 2007;1:CD003510. [PUBMED: 17253490] - PubMed
Watts 2004 {published data only}
-
- Watts DH, Balasubramanian R, Maupin RT Jr, Delke I, Dorenbaum A, Fiore S, et al. Maternal toxicity and pregnancy complications in human immunodeficiency virus‐infected women receiving antiretroviral therapy: PACTG 316. American journal of obstetrics and gynecology 2004;190(2):506‐16. [PUBMED: 14981398] - PubMed
Watts 2004a {published data only}
-
- Watts DH, Covington DL, Beckerman K, Garcia P, Scheuerle A, Dominguez K, et al. Assessing the risk of birth defects associated with antiretroviral exposure during pregnancy. American journal of obstetrics and gynecology 2004;191(3):985‐92. [PUBMED: 15467577] - PubMed
Weinberg 2009 {published data only}
-
- Weinberg A, Forster‐Harwood J, McFarland EJ, Pappas J, Davies JK, Kinzie K, et al. Resistance to antiretrovirals in HIV‐infected pregnant women. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 2009;45(1):39‐42. [PUBMED: 19329355] - PubMed
Zorrilla 2007 {published data only}
-
- Zorrilla CD, Dyke R, Bardeguez A, Acosta EP, Smith B, Hughes MD, et al. Clinical response and tolerability to and safety of saquinavir with low‐dose ritonavir in human immunodeficiency virus type 1‐infected mothers and their infants. Antimicrobial agents and chemotherapy 2007;51(6):2208‐10. [PUBMED: 17420209] - PMC - PubMed
Additional references
Bond 2007
-
- Bond K, Horváth T, Harvey K, Wiysonge CS, Read JS. The Cochrane Library and mother‐to‐child transmission of HIV: an umbrella review. Evidence‐based Child Health: A Cochrane Review Journal 2007;2:4‐24.
Bryson 1992
-
- Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV‐1. The New England journal of medicine 1992; Vol. 327, issue 17:1246‐7. [PUBMED: 1406816] - PubMed
Bulterys 1997
-
- Bulterys M, Landesman S, Burns DN, Rubinstein A, Goedert JJ. Sexual behavior and injection drug use during pregnancy and vertical transmission of HIV‐1. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association 1997;15(1):76‐82. [PUBMED: 9215658] - PubMed
Connor 1994
-
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, et al. Reduction of maternal‐infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. The New England journal of medicine 1994;331(18):1173‐80. [PUBMED: 7935654] - PubMed
Cooper 2002
-
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV‐1‐infected women and prevention of perinatal HIV‐1 transmission. Journal of acquired immune deficiency syndromes (1999) 2002;29(5):484‐94. [PUBMED: 11981365] - PubMed
De Cock 2000
-
- Cock KM, Fowler MG, Mercier E, Vincenzi I, Saba J, Hoff E, et al. Prevention of mother‐to‐child HIV transmission in resource‐poor countries: translating research into policy and practice. JAMA : the journal of the American Medical Association 2000;283(9):1175‐82. [PUBMED: 10703780] - PubMed
European Collaborative Study (ECS) 1999
-
- European Collaborative Study. Maternal viral load and vertical transmission of HIV‐1: an important factor but not the only one. The European Collaborative Study. AIDS (London, England) 1999;13(11):1377‐85. [PUBMED: 10449292] - PubMed
European Collaborative Study (ECS) 2000
-
- European Collaborative Study. Combination antiretroviral therapy and duration of pregnancy. AIDS (London, England) 2000;14(18):2913‐20. [PUBMED: 11398741] - PubMed
European Mode of Delivery Collaboration‐ EMDC 1999
-
- European Mode of Delivery Collaboration. Elective caesarean‐section versus vaginal delivery in prevention of vertical HIV‐1 transmission: a randomised clinical trial. The European Mode of Delivery Collaboration. Lancet 1999;353(9158):1035‐9. [PUBMED: 10199349] - PubMed
Fitzgerald 2009
-
- Fitzgerald D and the GHESKIO Centers, Haiti. A randomized clinical trial of early versus standard antiretoviral therapy for HIV‐infected patients with a CD4 T cell count of 200‐350 cells/mL (CIPRAHT001). International AIDS Society Conference. Capetown, South Africa;July 2009:Abstract WESY201.
Floridia 2008
-
- Floridia M, Ravizza M, Bucceri A, Lazier L, Vigano A, Alberico S, et al. Factors influencing gestational age‐adjusted birthweight in a national series of 600 newborns from mothers with HIV. HIV clinical trials 2008;9(5):287‐97. [PUBMED: 18977717] - PubMed
Guay 1999
-
- Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single‐dose nevirapine compared with zidovudine for prevention of mother‐to‐child transmission of HIV‐1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999;354(9181):795‐802. [PUBMED: 10485720] - PubMed
Higgins 2008
-
- Higgins JPT, Green S. Cochrane handbook for systematic reviews. Hoboken, NJ;Wiley‐Blackwell:2008.
Hitti 2004
-
- Hitti J, Frenkel LM, Stek AM, Nachman SA, Baker D, Gonzalez‐Garcia A, et al. Maternal toxicity with continuous nevirapine in pregnancy: results from PACTG 1022. Journal of acquired immune deficiency syndromes (1999) 2004;36(3):772‐6. [PUBMED: 15213559] - PubMed
Horvath 2009
International Perinatal HIV Group (IPHG) 1999
-
- International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1‐‐a meta‐analysis of 15 prospective cohort studies. The International Perinatal HIV Group. The New England journal of medicine 1999;340(13):977‐87. [PUBMED: 10099139] - PubMed
International Perinatal HIV Group (IPHG) 2001
-
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV‐1: a meta‐analysis from 15 prospective cohort studies. AIDS (London, England) 2001;15(3):357‐68. [PUBMED: 11273216] - PubMed
Ioannidis 2001
-
- Ioannidis JP, Abrams EJ, Ammann A, Bulterys M, Goedert JJ, Gray L, et al. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus loads <1000 copies/ml. The Journal of infectious diseases 2001;183(4):539‐45. [PUBMED: 11170978] - PubMed
Kwiek 2006
Lallemant 2000
-
- Lallemant M, Jourdain G, Coeur S, Kim S, Koetsawang S, Comeau AM, et al. A trial of shortened zidovudine regimens to prevent mother‐to‐child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. The New England journal of medicine 2000;343(14):982‐91. [PUBMED: 11018164] - PubMed
Langston 1995
-
- Langston C, Lewis DE, Hammill HA, Popek EJ, Kozinetz CA, Kline MW, et al. Excess intrauterine fetal demise associated with maternal human immunodeficiency virus infection. The Journal of infectious diseases 1995;172(6):1451‐60. [PUBMED: 7594702] - PubMed
McIntyre 2009
Mofenson 1997
-
- Mofenson LM. Mother‐child HIV‐1 transmission: Timing and determinants. Obstetrics and gynecology clinics of North America 1997;24(4):759‐84. [PUBMED: 9430166] - PubMed
Mofenson 1999
-
- Mofenson LM, Lambert JS, Stiehm ER, Bethel J, Meyer WA 3rd, Whitehouse J, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. The New England journal of medicine 1999;341(6):385‐93. [PUBMED: 10432323] - PubMed
Moodley 2003
-
- Moodley D, Moodley J, Coovadia H, Gray G, McIntyre J, Hofmyer J, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother‐to‐child transmission of human immunodeficiency virus type 1. The Journal of infectious diseases 2003;187(5):725‐35. [PUBMED: 12599045] - PubMed
Nduati 2000
-
- Nduati R, John G, Mbori‐Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV‐1: a randomized clinical trial. JAMA : the journal of the American Medical Association 2000;283(9):1167‐74. [PUBMED: 10703779] - PubMed
Newcastle‐Ottawa
-
- Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta‐analyses. Ottawa Health Research Institute. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
Nicoll 1995
-
- Nicoll A, Newell ML, Praag E, Perre P, Peckham C. Infant feeding policy and practice in the presence of HIV‐1 infection. AIDS (London, England) 1995; Vol. 9, issue 2:107‐19. [PUBMED: 7718182] - PubMed
Perinatal HIV Guidelines Working Group, 2009
-
- The Perinatal HIV Guidelines Working Group. Public Health Service Task Force Recommendations for Use of Antiretroviral Drugs in Pregnant HIV‐Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. April 29, 2009 pp 1‐90;Available at http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf:Accessed January 13, 2010.
PETRA 2002
-
- Petra Study Group. Efficacy of three short‐course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV‐1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double‐blind, placebo‐controlled trial. Lancet 2002;359(9313):1178‐86. [PUBMED: 11955535] - PubMed
Rasheed 1996
-
- Rasheed S, Li Z, Xu D, Kovacs A. Presence of cell‐free human immunodeficiency virus in cervicovaginal secretions is independent of viral load in the blood of human immunodeficiency virus‐infected women. American journal of obstetrics and gynecology 1996;175(1):122‐9. [PUBMED: 8694037] - PubMed
Read 2005
-
- Read JS, Newell MK. Efficacy and safety of cesarean delivery for prevention of mother‐to‐child transmission of HIV‐1. Cochrane database of systematic reviews (Online) 2005;1:CD005479. [PUBMED: 16235405] - PubMed
Richardson 2003
Schulte 2007
-
- Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler MG. Declines in low birth weight and preterm birth among infants who were born to HIV‐infected women during an era of increased use of maternal antiretroviral drugs: Pediatric Spectrum of HIV Disease, 1989‐2004. Pediatrics 2007;119(4):e900‐6. [PUBMED: 17353299] - PubMed
Shaffer 1999
-
- Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. Short‐course zidovudine for perinatal HIV‐1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet 1999;353(9155):773‐80. [PUBMED: 10459957] - PubMed
Szyld 2006
-
- Szyld EG, Warley EM, Freimanis L, Gonin R, Cahn PE, Calvet GA, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS (London, England) 2006;20(18):2345‐53. [PUBMED: 17117021] - PubMed
Taha (NVAZ) 2003
-
- Taha TE, Kumwenda NI, Gibbons A, Broadhead RL, Fiscus S, Lema V, et al. Short postexposure prophylaxis in newborn babies to reduce mother‐to‐child transmission of HIV‐1: NVAZ randomised clinical trial. Lancet 2003;362(9391):1171‐7. [PUBMED: 14568737] - PubMed
Taha 2004
-
- Taha TE, Kumwenda NI, Hoover DR, Fiscus SA, Kafulafula G, Nkhoma C, et al. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA : the journal of the American Medical Association 2004;292(2):202‐9. [PUBMED: 15249569] - PubMed
Taha 2007
-
- Taha TE, Hoover DR, Kumwenda NI, Fiscus SA, Kafulafula G, Nkhoma C, et al. Late postnatal transmission of HIV‐1 and associated factors. The Journal of infectious diseases 2007;196(1):10‐4. [PUBMED: 17538877] - PubMed
Thior 2006
-
- Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother‐to‐child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA : the journal of the American Medical Association 2006;296(7):794‐805. [PUBMED: 16905785] - PubMed
Townsend 2008
-
- Townsend CL, Cortina‐Borja M, Peckham CS, Ruiter A, Lyall H, Tookey PA. Low rates of mother‐to‐child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000‐2006. AIDS (London, England) 2008;22(8):973‐81. [PUBMED: 18453857] - PubMed
Tuomala 2002
-
- Tuomala RE, Shapiro DE, Mofenson LM, Bryson Y, Culnane M, Hughes MD, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. The New England journal of medicine 2002;346(24):1863‐70. [PUBMED: 12063370] - PubMed
UNAIDS 2009
-
- Joint United Nations Programme on HIV/AIDS. 2009 AIDS Epidemic Update. Geneva, Geneva: 2009;1:1‐100.
Watts 2004
-
- Watts DH, Balasubramanian R, Maupin RT Jr, Delke I, Dorenbaum A, Fiore S, et al. Maternal toxicity and pregnancy complications in human immunodeficiency virus‐infected women receiving antiretroviral therapy: PACTG 316. American journal of obstetrics and gynecology 2004;190(2):506‐16. [PUBMED: 14981398] - PubMed
WHO (Infant Feeding) 2009
-
- WHO Infant Feeding Collaborative Study Team. Rapid advice: infant feeding in the context of HIV. Geneva October 22‐23, 2009;1:1‐30.
WHO 2006
-
- WHO MTCT Collaborative Study Team. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access. Geneva June 28‐29, 2005;1:1‐87.
WHO 2009
-
- WHO MTCT Collaborative Study Team. Rapid Advice: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva October 19‐21, 2009;1:1‐26.
Wiysonge 2005
-
- Wiysonge CS, Shey MS, Shang JD, Sterne JA, Brocklehurst P. Vaginal disinfection for preventing mother‐to‐child transmission of HIV infection. Cochrane database of systematic reviews (Online) 2005;1(4):CD003651. [PUBMED: 16235334] - PubMed
Wiysonge 2005a
-
- Wiysonge CS, Shey MS, Sterne JA, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane database of systematic reviews (Online) 2005;1(4):CD003648. [PUBMED: 16235332] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

