The effects of alpha-helical structure and cyanylated cysteine on each other
- PMID: 20297787
- PMCID: PMC2851192
- DOI: 10.1021/jp101447r
The effects of alpha-helical structure and cyanylated cysteine on each other
Abstract
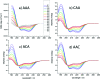
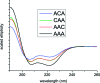
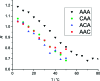
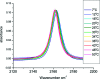
Beta-thiocyanatoalanine, or cyanylated cysteine, is an artificial amino acid that can be introduced at solvent-exposed cysteine residues in proteins via chemical modification. Its facile post-translational synthesis means that it may find broad use in large protein systems as a probe of site-specific structure and dynamics. The C[triple bond]N stretching vibration of this artificial side chain provides an isolated infrared chromophore. To test both the perturbative effect of this side chain on local secondary structure and its sensitivity to structural changes, three variants of a model water-soluble alanine-repeat helix were synthesized containing cyanylated cysteine at different sites. The cyanylated cysteine side chain is shown to destabilize, but not completely disrupt, the helical structure of the folded peptide when substituted for alanine. In addition, the [triple bond]N stretching bandwidth of the artificial side chain is sensitive to the helix-coil structural transition. These model system results indicate that cyanylated cysteine can be placed into protein sequences with a native helical propensity without destroying the helix, and further that the CN probe may be able to report local helix formation events even when it is water-exposed in both the ordered and disordered conformational states. These results indicate that cyanylated cysteine could be a widely useful probe of structure-forming events in proteins with large in vitro structural distributions.
Figures
Similar articles
-
Using infrared spectroscopy of cyanylated cysteine to map the membrane binding structure and orientation of the hybrid antimicrobial peptide CM15.Biochemistry. 2011 Dec 27;50(51):11097-108. doi: 10.1021/bi200903p. Epub 2011 Dec 2. Biochemistry. 2011. PMID: 22103476 Free PMC article.
-
Probing structural transitions in the intrinsically disordered C-terminal domain of the measles virus nucleoprotein by vibrational spectroscopy of cyanylated cysteines.Biophys J. 2010 Sep 8;99(5):1676-83. doi: 10.1016/j.bpj.2010.06.060. Biophys J. 2010. PMID: 20816082 Free PMC article.
-
Monitoring structural transitions in IDPs by vibrational spectroscopy of cyanylated cysteine.Methods Mol Biol. 2012;895:245-70. doi: 10.1007/978-1-61779-927-3_17. Methods Mol Biol. 2012. PMID: 22760324
-
Cyanylated Cysteine: A Covalently Attached Vibrational Probe of Protein-Lipid Contacts.J Phys Chem Lett. 2010 Mar 4;1(5):850-855. doi: 10.1021/jz1000177. Epub 2010 Feb 12. J Phys Chem Lett. 2010. PMID: 20228945 Free PMC article.
-
[A turning point in the knowledge of the structure-function-activity relations of elastin].J Soc Biol. 2001;195(2):181-93. J Soc Biol. 2001. PMID: 11727705 Review. French.
Cited by
-
Using infrared spectroscopy of cyanylated cysteine to map the membrane binding structure and orientation of the hybrid antimicrobial peptide CM15.Biochemistry. 2011 Dec 27;50(51):11097-108. doi: 10.1021/bi200903p. Epub 2011 Dec 2. Biochemistry. 2011. PMID: 22103476 Free PMC article.
-
Two-dimensional infrared spectroscopy of azido-nicotinamide adenine dinucleotide in water.J Chem Phys. 2011 Aug 7;135(5):055106. doi: 10.1063/1.3623418. J Chem Phys. 2011. PMID: 21823737 Free PMC article.
-
Probing structural transitions in the intrinsically disordered C-terminal domain of the measles virus nucleoprotein by vibrational spectroscopy of cyanylated cysteines.Biophys J. 2010 Sep 8;99(5):1676-83. doi: 10.1016/j.bpj.2010.06.060. Biophys J. 2010. PMID: 20816082 Free PMC article.
-
Site-Specific Spectroscopic Reporters of the Local Electric Field, Hydration, Structure, and Dynamics of Biomolecules.J Phys Chem Lett. 2011 Sep 23;2:2598-2609. doi: 10.1021/jz201161b. J Phys Chem Lett. 2011. PMID: 22003429 Free PMC article.
-
A thiocyanopalladation/carbocyclization transformation identified through enzymatic screening: stereocontrolled tandem C-SCN and C-C bond formation.Chem Sci. 2017 Dec 1;8(12):8050-8060. doi: 10.1039/c7sc04083k. Epub 2017 Oct 3. Chem Sci. 2017. PMID: 29568453 Free PMC article.
References
-
- Decatur S. M.; Antonic J. J. Am. Chem. Soc. 1999, 121, 11914–11915.
-
- Huang R.; Kubelka J.; Barber-Armstrong W.; Silva R. A. G. D.; Decatur S. M.; Keiderling T. A. J. Am. Chem. Soc. 2004, 126, 2346–2354. - PubMed
-
- Setnicka V.; Huang R.; Thomas C. L.; Etienne M. A.; Kubelka J.; Hammer R. P.; Keiderling T. A. J. Am. Chem. Soc. 2005, 127, 4992–4993. - PubMed
-
- Brewer S. H.; Song B. B.; Raleigh D. P.; Dyer R. B. Biochemistry 2007, 46, 3279–3285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

