The productive merger of iodonium salts and organocatalysis: a non-photolytic approach to the enantioselective alpha-trifluoromethylation of aldehydes
- PMID: 20297822
- PMCID: PMC2880471
- DOI: 10.1021/ja100748y
The productive merger of iodonium salts and organocatalysis: a non-photolytic approach to the enantioselective alpha-trifluoromethylation of aldehydes
Abstract
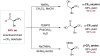
An enantioselective organocatalytic alpha-trifluoromethylation of aldehydes has been accomplished using a commercially available, electrophilic trifluoromethyl source. The merging of Lewis acid and organocatalysis provides a new strategy for the enantioselective construction of trifluoromethyl stereogenicity, an important chiral synthon for pharmaceutical, materials, and agrochemical applications. This mild and operationally simple protocol allows rapid access to enantioenriched alpha-trifluoromethylated aldehydes through a nonphotolytic pathway.
Figures
References
-
- Chambers RD. Fluorine in Organic Chemistry. Wiley; New York: 1973.
- Banks RE, Smart BE, Tatlow JC. Organofluorine Chemistry: Principles and Commercial Applications. Plenum Press; New York: 1994.
- Filler R, Kobayashi Y, Yagupolskii LM. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications. Elsevier; New York: 1993.
- Müller K, Faeh C, Diederich F. Science. 2007;317:1881. - PubMed
- Pursor S, Moore PR, Swallow S, Gouverneur V. Chem Soc Rev. 2008;37:320. - PubMed
-
-
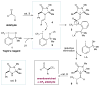
For seminal reports of Togni’s reagent and its applications, see: Eisenberger P, Gischig S, Togni A. Chem Eur J. 2006;12:2579.Kieltsch I, Eisenberger P, Togni A. Angew Chem, Int Ed. 2007;46:754.Eisenberger P, Kieltsch I, Armanino N, Togni A. Chem Commun. 2008:1575.Kieltsch I, Eisenberger P, Stanek K, Togni A. Chimia. 2008;62:260.Koller R, Stanek K, Stolz D, Aardoom R, Niedermann K, Togni A. Angew Chem, Int Ed. 2009;48:4332.
-
-
-
For seminal contributions towards other electrophilic trifluoromethylating sources, see: Yagupolskii LM, Kondratenko NV, Timofeeva GN. J Org Chem USSR (Engl Transl) 1984;20:103.Umemoto T, Ishihara S. Tetrahedron Lett. 1990;31:3579.Umemoto T, Ishihara S. J Am Chem Soc. 1993;115:2156.Umemoto T. Chem Rev. 1996;96:1757.
-
-
-
Imidazolidinone 3 can be purchased from Sigma-Aldrich as the hydrochloric acid salt (CAS: 278173-23-2). The hydrochloric acid salt was converted to the trifluoroacetic acid salt before use in this protocol.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources