Targeting apoptosis pathway with natural terpenoids: implications for treatment of breast and prostate cancer
- PMID: 20298150
- PMCID: PMC3306610
- DOI: 10.2174/138945010791170842
Targeting apoptosis pathway with natural terpenoids: implications for treatment of breast and prostate cancer
Abstract
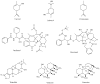
Terpenoids represent a large and diverse class of naturally occurring compounds found in a variety of fruits, vegetables and medicinal plants. Structurally some of the terpenoids are similar to human hormones. A diet rich in terpenoids is inversely related with the risk of chronic diseases including cancers. Breast and prostate cancers are hormone-related diseases and the second leading cause of female and male cancer mortality. Diterpenoid paclitaxel, and its semi-synthetic analogue docetaxel, have entered clinical use against established breast and prostate cancers. Here we reviewed potential molecular targets and biological properties of natural terpenoids, including monoterpenoids, diterpenoids, triterpenoids and tetraterpenoids, and their applications in treatment of human breast and prostate cancers. These terpenoids are able to inhibit tumor cell proliferation and induce tumor cell death by inhibiting multiple cancer-specific targets including the proteasome, NF-kappaB, and antiapoptotic protein Bcl-2. The efficacy of these terpenoids against breast or prostate cancer cells, as demonstrated in pre-clinical studies support clinical application of these naturally occurring terpenoids in treatment of hormone-related human cancers.
Figures


Similar articles
-
Insights into the mechanism of natural terpenoids as NF-κB inhibitors: an overview on their anticancer potential.Exp Oncol. 2016 Sep;38(3):158-68. Exp Oncol. 2016. PMID: 27685522 Review.
-
Terpenoids: natural products for cancer therapy.Expert Opin Investig Drugs. 2012 Dec;21(12):1801-18. doi: 10.1517/13543784.2012.727395. Epub 2012 Oct 23. Expert Opin Investig Drugs. 2012. PMID: 23092199 Review.
-
Inhibition of tumor progression by naturally occurring terpenoids.Pharm Biol. 2011 Oct;49(10):995-1007. doi: 10.3109/13880209.2011.559476. Pharm Biol. 2011. PMID: 21936626 Review.
-
Natural Terpenoids Against Female Breast Cancer: A 5-year Recent Research.Curr Med Chem. 2018;25(27):3162-3213. doi: 10.2174/0929867325666180214110932. Curr Med Chem. 2018. PMID: 29446727 Review.
-
Dietary terpenoids and prostate cancer chemoprevention.Front Biosci. 2008 May 1;13:3457-69. doi: 10.2741/2940. Front Biosci. 2008. PMID: 18508447 Free PMC article.
Cited by
-
Bromoditerpenes from the Red Seaweed Sphaerococcus coronopifolius as Potential Cytotoxic Agents and Proteasome Inhibitors and Related Mechanisms of Action.Mar Drugs. 2022 Oct 20;20(10):652. doi: 10.3390/md20100652. Mar Drugs. 2022. PMID: 36286475 Free PMC article.
-
Targeting cancer with sesterterpenoids: the new potential antitumor drugs.J Nat Med. 2015 Jul;69(3):255-66. doi: 10.1007/s11418-015-0911-y. Epub 2015 Apr 19. J Nat Med. 2015. PMID: 25894074 Free PMC article. Review.
-
Future in the Past: Azorella glabra Wedd. as a Source of New Natural Compounds with Antiproliferative and Cytotoxic Activity on Multiple Myeloma Cells.Int J Mol Sci. 2018 Oct 26;19(11):3348. doi: 10.3390/ijms19113348. Int J Mol Sci. 2018. PMID: 30373165 Free PMC article.
-
Identification of Gene Biomarkers for Tigilanol Tiglate Content in Fontainea picrosperma.Molecules. 2022 Jun 21;27(13):3980. doi: 10.3390/molecules27133980. Molecules. 2022. PMID: 35807225 Free PMC article.
-
Oleanane triterpenoids in the prevention and therapy of breast cancer: current evidence and future perspectives.Phytochem Rev. 2014 Dec;13(4):793-810. doi: 10.1007/s11101-014-9337-5. Phytochem Rev. 2014. PMID: 25395898 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
-
- Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–33. - PubMed
-
- Tharakan R, Lepont P, Singleton D, Kumar R, Khan S. Phosphorylation of estrogen receptor alpha, serine residue 305 enhances activity. Mol Cell Endocrinol. 2008;295:70–8. - PubMed
-
- Irvin WJ, Jr, Carey LA. What is triple-negative breast cancer? Eur J Cancer. 2008;44:2799–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
