Development of proteoglycan-induced arthritis depends on T cell-supported autoantibody production, but does not involve significant influx of T cells into the joints
- PMID: 20298547
- PMCID: PMC2888192
- DOI: 10.1186/ar2954
Development of proteoglycan-induced arthritis depends on T cell-supported autoantibody production, but does not involve significant influx of T cells into the joints
Abstract
Introduction: Inflammatory joint destruction in rheumatoid arthritis (RA) may be triggered by autoantibodies, the production of which is supported by autoreactive T cells. Studies on RA and animal models of the disease suggest that T cells recruited in the joints can locally initiate or propagate arthritis. Herein, we investigated the role of joint-homing versus lymphoid organ-homing T cells in the development of proteoglycan-induced arthritis (PGIA), an autoimmune model of RA.
Methods: To identify T cells migrating to the joints before and during development of autoimmune arthritis, we transferred fluorescence-labeled T cells, along with antigen-presenting cells, from BALB/c mice with PGIA to naïve syngeneic severe combined immunodeficient (SCID) mice. We then monitored the recruitment of donor T cells in the ankle joints and joint-draining lymph nodes of the recipients using in vivo two-photon microscopy and ex vivo detection methods. To limit T-cell access to the joints, we selectively depleted T cells in the blood circulation by treatment with FTY720, an inhibitor of lymphocyte egress from lymphoid organs. Reduction of T cell presence in both lymphoid organs and blood was achieved by injection of donor cells from which T cells were removed prior to transfer. T and B cells were quantitated by flow cytometry, and antigen (PG)-specific responses were assessed by cell proliferation and serum antibody assays.
Results: Despite development of adoptively transferred arthritis in the recipient SCID mice, we found very few donor T cells in their joints after cell transfer. Treatment of recipient mice with FTY720 left the T-cell pool in the lymphoid organs intact, but reduced T cells in both peripheral blood and joints. However, FTY720 treatment failed to inhibit PGIA development. In contrast, arthritis was not seen in recipient mice after transfer of T cell-depleted cells from arthritic donors, and serum autoantibodies to PG were not detected in this group of mice.
Conclusions: Our results suggest that antigen-specific T cells, which home to lymphoid organs and provide help to B cells for systemic autoantibody production, play a greater role in the development and progression of autoimmune arthritis than the small population of T cells that migrate to the joints.
Figures
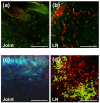



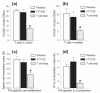
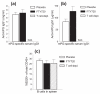
Comment in
-
Arthritis: where are the T cells?Arthritis Res Ther. 2010;12(3):122. doi: 10.1186/ar3008. Epub 2010 Jun 3. Arthritis Res Ther. 2010. PMID: 20537200 Free PMC article.
References
-
- McCarty DJ. In: Arthritis and Allied Conditions: A Textbook of Rheumatology. McCarty DJ, Koopman WJ, editor. Philadelphia, London: Lea & Fabiger; 1993. Clinical picture of rheumatoid arthritis; pp. 781–809.
-
- Firestein GS. In: Kelley's Textbook of Rheumatology. Ruddy S, Harris ED, Sledge CB, Kelley WN, editor. Philadelphia, PA: W.B. Saunders Co; 2005. Rheumatoid arthritis: etiology and pathogenesis of rheumatoid arthritis; pp. 996–1045.
-
- Nielen MM, van Schaardenburg D, Reesink HW, Twisk JW, Stadt RJ van de, Horst-Bruinsma IE van der, de Koning MH, Habibuw MR, Dijkmans BA. Simultaneous development of acute phase response and autoantibodies in preclinical rheumatoid arthritis. Ann Rheum Dis. 2006;65:535–537. doi: 10.1136/ard.2005.040659. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

