Krüppel-like factor 5 is a crucial mediator of intestinal tumorigenesis in mice harboring combined ApcMin and KRASV12 mutations
- PMID: 20298593
- PMCID: PMC2856552
- DOI: 10.1186/1476-4598-9-63
Krüppel-like factor 5 is a crucial mediator of intestinal tumorigenesis in mice harboring combined ApcMin and KRASV12 mutations
Abstract
Background: Both mutational inactivation of the adenomatous polyposis coli (APC) tumor suppressor gene and activation of the KRAS oncogene are implicated in the pathogenesis of colorectal cancer. Mice harboring a germline ApcMin mutation or intestine-specific expression of the KRASV12 gene have been developed. Both mouse strains develop spontaneous intestinal tumors, including adenoma and carcinoma, though at a different age. The zinc finger transcription factor Krüppel-like factor 5 (KLF5) has previously been shown to promote proliferation of intestinal epithelial cells and modulate intestinal tumorigenesis. Here we investigated the in vivo effect of Klf5 heterozygosity on the propensity of ApcMin/KRASV12 double transgenic mice to develop intestinal tumors.
Results: At 12 weeks of age, ApcMin/KRASV12 mice had three times as many intestinal tumors as ApcMin mice. This increase in tumor number was reduced by 92% in triple transgenic ApcMin/KRASV12/Klf5+/- mice. The reduction in tumor number in ApcMin/KRASV12/Klf5+/- mice was also statistically significant compared to ApcMin mice alone, with a 75% decrease. Compared with ApcMin/KRASV12, tumors from both ApcMin/KRASV12/Klf5+/- and ApcMin mice were smaller. In addition, tumors from ApcMin mice were more distally distributed in the intestine as contrasted by the more proximal distribution in ApcMin/KRASV12 and ApcMin/KRASV12/Klf5+/- mice. Klf5 levels in the normal-appearing intestinal mucosa were higher in both ApcMin and ApcMin/KRASV12 mice but were attenuated in ApcMin/KRASV12/Klf5+/- mice. The levels of beta-catenin, cyclin D1 and Ki-67 were also reduced in the normal-appearing intestinal mucosa of ApcMin/KRASV12/Klf5+/- mice when compared to ApcMin/KRASV12 mice. Levels of pMek and pErk1/2 were elevated in the normal-appearing mucosa of ApcMin/KRASV12 mice and modestly reduced in ApcMin/KRASV12/Klf5+/- mice. Tumor tissues displayed higher levels of both Klf5 and beta-catenin, irrespective of the mouse genotype from which tumors were derived.
Conclusions: Results of the current study confirm the cumulative effect of Apc loss and oncogenic KRAS activation on intestinal tumorigenesis. The drastic reduction in tumor number and size due to Klf5 heterozygosity in ApcMin/KRASV12 mice indicate a critical function of KLF5 in modulating intestinal tumor initiation and progression.
Figures

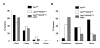
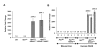






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

